
1
Acetylcysteine (N-acetylcysteine, NAC) for the
management of non-acetaminophen-induced acute
liver failure
Submitted by:
Jill M. Pulley, MBA, Executive Director, and Rebecca Jerome, MLIS, MPH,
Manager, Translational Research
Vanderbilt Institute for Clinical and Translational Research
Vanderbilt University Medical Center, Nashville Tennessee, USA
(see Appendix 1 for a list of additional contributors)
Submission Date: November 30, 2020
Project | Remedi aims to uncover new therapeutic uses for hundreds of medicines
on the Essential Medicines List, seek approval to add them to the EML, and amplify
availability of new uses to benefit priority populations.
2
Contents
1. Summary statement of the proposal for inclusion, change or deletion ........................................................ 4
2. Relevant WHO technical department and focal point (if applicable).
3. Name of organization(s) consulted and/or supporting the application. ........................................................ 6
4. International Nonproprietary Name (INN) and Anatomical Therapeutic Chemical (ATC) code of the
medicine. ............................................................................................................................................................ 6
5. Dose forms(s) and strength(s) proposed for inclusion ................................................................................... 6
6. Whether listing is requested as an individual medicine or as representative of a pharmacological class. ... 6
7. Treatment details (requirements for diagnosis, treatment and monitoring). ............................................... 7
8. Information supporting the public health relevance. .................................................................................... 8
9. Review of benefits: summary of evidence of comparative effectiveness. ..................................................... 9
Identification of clinical evidence (search strategy, systematic reviews identified, reasons for
selection/exclusion of particular data) ........................................................................................................... 9
Summary of available data (appraisal of quality, outcome measures, summary of results) ......................... 9
Summary of available estimates of comparative effectiveness ................................................................... 12
10. Review of harms and toxicity: summary of evidence of safety. ................................................................. 12
Estimate of total patient exposure to date: ................................................................................................. 12
Description of the adverse effects/reactions when used in non-acetaminophen induced acute liver
failure: ........................................................................................................................................................... 12
Description of the adverse effects/reactions and estimates of their frequency (drawn from the broader
NAC literature on human use) ...................................................................................................................... 12
Summary of available data ........................................................................................................................... 13
Identification of variation in safety that may relate to health systems and patient factors ....................... 15
11. Summary of available data on comparative cost and cost-effectiveness of the medicine. ....................... 16
12. Summary of regulatory status and market availability of the medicine. ................................................... 16
13. Availability of pharmacopoeial standards (British Pharmacopoeia, International Pharmacopoeia, United
States Pharmacopoeia, European Pharmacopeia). Summary of available data on comparative cost and cost-
effectiveness of the medicine. ......................................................................................................................... 17
Literature summaries: non-acetaminophen acute liver failure, organized by precipitating
exposure/condition .......................................................................................................................................... 18
LITERATURE SUMMARY: Evidence describing use of NAC in general non-acetaminophen-induced acute
liver injury ..................................................................................................................................................... 18
LITERATURE SUMMARY: Evidence describing use of NAC in heatstroke-associated acute liver injury ....... 24
LITERATURE SUMMARY: Evidence describing use of NAC in alcohol poisoning-associated acute liver injury
...................................................................................................................................................................... 25
LITERATURE SUMMARY: Evidence describing use of NAC in mushroom toxin-induced acute liver injury.. 25
3
LITERATURE SUMMARY: Evidence describing use of NAC in virus-associated acute liver injury (hepatitis A,
hepatitis B, dengue fever) ............................................................................................................................ 26
14. Comprehensive reference list and in-text citations. .................................................................................. 31
Appendix 1: Additional contributors ................................................................................................................ 41
Appendix 2: Evidence describing hepatic effects of N- acetylcysteine in other conditions (excluding
acetaminophen toxicity) ................................................................................................................................... 42
Appendix 3: Summary of adverse events reported in systematic reviews, by indication................................ 51

4
1. Summary statement of the proposal for inclusion, change or deletion
We propose a new listing to the EML to add an additional use of a medicine already on the EML, N-
acetylcysteine (NAC). The new indication is for the management of non-acetaminophen-induced
acute liver failure (ALF) caused by etiologies that deplete glutathione (see Figure 1). This
indication leverages a sound foundation of trial and observational evidence supporting the safety and
utility of NAC in preventing further progression of liver failure in adults and children. This indication
includes a range of etiologies for ALF with known connection to glutathione depletion which leads to
hepatic injury; NAC replenishes intracellular glutathione and exerts antioxidant effects which help to
ameliorate the adverse consequences of the hepatic insult and its sequelae. This request is being sought
for the complementary EML, as patients with ALF are typically cared for in a hospital/specialized setting.
Generally, N-acetylcysteine (NAC) is known via preclinical and clinical studies for its hepatoprotective
effects by increasing intracellular glutathione particularly in the liver and by its antioxidant properties
which counteract oxidative stress and inflammation.(1) NAC has been in widespread use since the 1960s
and has been proven to be safe and well tolerated; its use as an antidote for acetaminophen toxicity (a
use in which oral and intravenous NAC have been shown to be equally effective in preventing and
minimizing hepatotoxicity), and is already represented on the EML for this use. Based on similar
mechanisms, NAC shows promise in protecting the liver against the effects of and response to
insults precipitating non-acetaminophen induced acute liver injury due to glutathione depletion,
including virus-induced acute hepatic failure; mushroom toxin-induced liver failure; acute
alcoholic hepatitis; and heat stroke-induced ALF (Figure 1). In addition to the range of studies
reporting the benefit and safety of NAC use in these indications (see section 9 and 10, Literature
Summary table), a body of literature describing NAC use in heterogeneous populations of non-
acetaminophen induced ALF(2–4) further supports this new indication for NAC. (see Literature
Summary section for a synthesis of relevant systematic reviews, trials, and observational studies).
Briefly, various insults (e.g. hepatitis A virus, dengue virus, toxic mushroom consumption, excess alcohol
intake, heat stroke) directly deplete glutathione, which is a necessary enzyme for proper liver
function. Each of these etiologies for ALF has supporting data indicating that glutathione depletion plays
an important role in development of ALF; the mechanisms of acute liver dysfunction and failure in these
conditions are believed to result directly from hepatocyte apoptosis/necrosis, hypoxic damage due to
impaired liver perfusion resulting from fluid leakage, as well as oxidative stress and immune mediated
injury. (5–17) NAC, through enhancing glutathione S-transferase activity, affects several of these
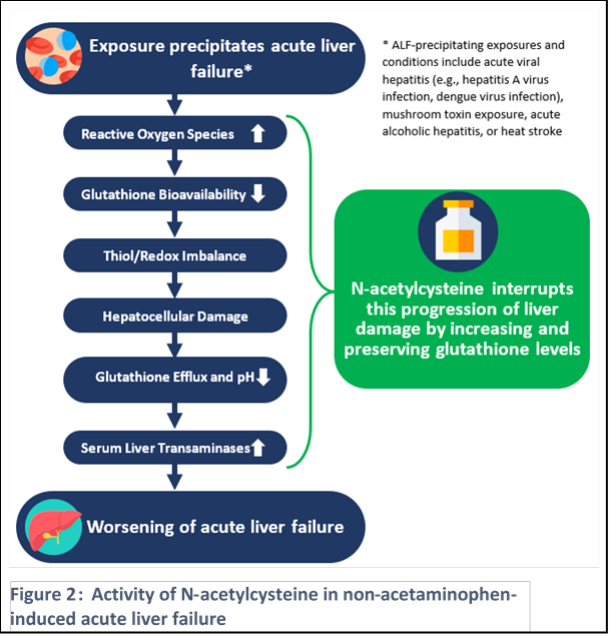
5
mechanisms (Figure 2).(1,18–21) In addition, NAC has antioxidative, anti-inflammatory, and
vasodilatory effects,(22) which can help counteract the adverse effects of impaired liver perfusion and
reducing hepatocytes apoptosis due to oxidative stress and immune-mediated injury.
While ALF remains relatively rare, it
affects children and adults across
the world and confers significant
morbidity and mortality. (23,24)
Care for ALF associated with these
etiologies is supportive in nature,
with no targeted options for
minimizing further injury to the
liver. To address an unmet medical
need with an existing, safe therapy,
we propose a new use for NAC in the
treatment of ALF caused by
hepatitis A, dengue virus, heat
stroke, acute alcohol poisoning, and
mushroom toxicity. NAC should be
administered to affected patients as
soon as possible based on presence
of hepatic injury (i.e. laboratory data
indicating increase in liver function
test results). The goal of this
strategy, based on the evidence
described below, is to prevent or
limit severity of acute liver failure
and related morbidity and
mortality.
The literature describing clinical use of NAC in general non-acetaminophen-induced ALF patients, as
well as those with ALF due to heat stroke, acute alcoholic hepatitis, mushroom poisoning, or acute viral
hepatitis, supports the safety and efficacy of this therapeutic approach in complementing usual
supportive care for patients affected by these types of ALF. The literature indicates that use of NAC
represents at least a significant incremental gain over supportive care alone, with a reasonable
expectation of direct effects on morbidity, including averting the need for transplantation in some
patients. In addition, with its long-standing history of use in acetaminophen-induced acute liver injury,
NAC has a strong foundation of data supporting its safety in children and adults.
There is precedent for this approach with the use of NAC from past EML committee decision related to
use as an antidote to acetaminophen toxicity, as real-world data was deemed sufficiently compelling.
The relevant excerpt from 2008 review states: “…subsequent human investigations have consisted
mostly of observational studies due to ethical concerns of withholding a potential lifesaving treatment.
Thus, there are no randomized controlled trials that evaluate NAC therapy for prevention of
acetaminophen-induced hepatotoxicity. Likewise, no randomized efficacy trials have been conducted in
children. Many of the trials evaluate efficacy based on the outcomes of historical control patients.”(25)

6
2. Relevant WHO technical department and focal point (if applicable).
Department of Neglected Tropical Diseases
Other interested groups may include Alcohol, Drugs and Addictive Behaviors Unit, Global HIV Hepatitis
and STIs Programme
3. Name of organization(s) consulted and/or supporting the application.
Dr. Robert Wallis, MD; Chief Scientific Officer, AURUM was consulted and reviewed this submission.
Dr. Gordon Bernard, MD; Executive Vice President for Research, Vanderbilt University Medical Center
was consulted and reviewed this submission.
4. International Nonproprietary Name (INN) and Anatomical Therapeutic
Chemical (ATC) code of the medicine.
INN: Acetylcysteine
A05: Bile and liver therapy
5. Dose forms(s) and strength(s) proposed for inclusion
This request is for the inclusion of NAC in intravenous or oral form for the EML. [Acetylcysteine is the
nonproprietary name for the N-acetyl derivative of the naturally occurring amino acid, L-cysteine (N-
acetyl-L cysteine)]. NAC is a generic medicine and is widely available internationally. Regarding
formulation, the WHO 2008 review of use of NAC in pediatric acetaminophen toxicity notes that oral
administration is preferred when there are not contraindications to its use (e.g. aspiration, persistent
vomiting)(25); intravenous use is recommended in this guidance when fulminant hepatic failure is
present, thus we suggest following this recommendation for the new indication of NAC use in various
types of acute liver failure, with use of intravenous NAC. Oral NAC may be considered when the i.v.
formulation is not available. In its use in the overdose setting to prevent hepatotoxicity, both oral and
i.v. NAC regimens are commonly used and well-tolerated, with no significant differences in safety or
efficacy.
Availability is supported given NAC is already on EML (in both injectable and oral forms) with
strengths (Injection: 200 mg/mL in 10- mL ampoule; oral liquid: 10%; 20%) appropriate for the detailed
treatment approach described below in Section 7. While the existing evidence base on use of NAC in
various types of non-acetaminophen-induced liver failure represents some variation in dosage and
administration schedules, these plans generally paralleled the NAC strategy used in acetaminophen
overdose and are similar for patients with acute liver failure due to other causes.
6. Whether listing is requested as an individual medicine or as
representative of a pharmacological class.
Individual medicine
7
7. Treatment details (requirements for diagnosis, treatment and
monitoring).
NAC administration should be initiated intravenously in patients with significant acute liver injury as
soon as ALF is detected, typically via presence of one of the precipitating conditions (e.g. acute viral
hepatitis, heat stroke, dengue, acute alcoholic hepatitis, mushroom toxicity) combined with alterations
in clinical status and liver function tests indicating acute liver failure as per local clinical standards.
The recommended IV protocol described in a previous review by WHO, focused on NAC use in
paracetamol toxicity in pediatrics,(25) adapted to incorporate regimen provisions in the literature
describing use of NAC in non-acetaminophen ALF, includes:
• Loading dose: administer 150 mg/kg IV over 1 hour
• Maintenance: followed by 50 mg/kg over 4 hours, then 100 mg/kg over 16 hours, then
100 mg/kg/day until up to 7 days after initial start of NAC depending on clinical
response.
• Modified IV dosing in those weighing less than 40 kg is recommended to avoid fluid
overload.
Administration should continue for a minimum of three days but longer as needed based on
assessment of the patient’s clinical status, laboratory testing of liver function and related measures
such as international normalized ratio (INR), and the time course of the underlying medical condition
(e.g. mushroom toxicity follows a shorter time course than dengue fever, which has a longer disease
course). To avoid fluid overload, the volume of diluent should be reduced whenever clinically needed.
The literature does not indicate that the dose of NAC in infected patients with hepatic impairment should
be reduced. Reduced clearance of NAC was observed in seven patients affected by chronic liver disease
as compared with six healthy controls, suggesting that it is possible that cirrhotic patients may be at
increased risk of hypersensitivity reactions.(26) The existing NAC literature indicates that
hypersensitivity reactions may be managed by decreasing the infusion rate or discontinuing the infusion
altogether.
If IV NAC is not available/feasible, oral NAC could be substituted using the protocol noted in the WHO
NAC review,(25) 140 mg/kg followed in 4 hours by a maintenance dose of 70 mg/kg orally given every
4 hours for up to 5 days, tailored to the condition of the patient under treatment.
Use in Children: NAC has a well-established safety profile, including extensive safety data in children
due to its use in acetaminophen toxicity. Use of NAC in ALF associated with the indications described in
this application, which each may affect this age group, would be appropriate in children.
Use in Pregnancy: The US Food and Drug Administration lists NAC as a Pregnancy Category B agent,
noting: “Limited case reports of pregnant women exposed to acetylcysteine during various trimesters
did not report any adverse maternal, fetal or neonatal outcomes.”(27) No significant adverse effects
involving the mother or fetus were observed in a prospective comparative study (n=80) of oral NAC for
treatment of recurrent unexplained pregnancy loss;(28) an RCT of oral NAC in women with severe early
onset preeclampsia or HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets);(29) an
RCT of IV NAC in maternal chorioamnionitis;(30) and an RCT of oral NAC in pregnant women with low
antioxidant status.(31) A randomized, double-blind, placebo-controlled trial of oral NAC for prevention
of recurrent preterm birth found no major maternal or fetal adverse effects; approximately 11% of
participants discontinued NAC due to nausea and vomiting.(32)
8
8. Information supporting the public health relevance.
While a relatively rare condition, acute liver failure is a serious clinical condition irrespective of country
and region, with high morbidity, as well as high mortality in the absence of supportive clinical care and
potentially liver transplantation. (23,24) ALF affects all age groups, and the causes of ALF are
heterogeneous; as noted above, we focus this application on ALF subsets with known involvement of
glutathione, given the targeting of this protein by NAC.
Acute viral hepatitis infections are responsible for most ALF cases globally, with variation in causative
viral pathogen in various regions (e.g. hepatitis A, B, E; dengue virus). (33) Considering dengue virus as
one key cause of acute liver injury and failure, the data suggests notable impact in some regions. Among
an estimated 390 million people infected with dengue each year, the WHO further estimates that 500
000 people with severe dengue require hospitalization and there is a 2.5% case fatality annually.(34) In
addition, there are growing reports of links between climate variations and the emergence of “climate-
sensitive infectious diseases”, which would include all of the mosquito-borne diseases dengue,
chikungunya, and Zika,(35) suggesting the global burden could be worsening. In the last 50 years,
incidence has been reported to have increased 30-fold. Although only nine countries had experienced
severe dengue epidemics prior to 1970, the disease is now endemic in over 120 countries resulting in
~3.9 billion people are at risk of infection.(36) Further, liver injury and failure may complicate the
disease course in a significant portion of individuals affected by dengue infection; in an analysis of 347
patients hospitalized for dengue fever during one outbreak in Thailand, 63% (n=219) had hepatic
failure.(37) The WHO notes:(34) “Dengue is increasing at a higher rate than any other communicable
disease, with 400% increase over 13 years (2000-2013). Annual dengue incidence is estimated to be in
the order of 100 million symptomatic cases a year, with another ~300 million asymptomatic infections.
The greatest burden is seen in Asia (75%) followed by Latin America and Africa.”
Heat stroke is another important cause of ALF. Incidence is difficult to estimate globally due to lack of
an accepted system for capture and reporting. In the US, for example, one study estimated over 4100
emergency department visits per year for heat stroke, an annual national incidence rate of 1.34
visits/100,000 people; this analysis noted a case fatality rate of 3.4%.(38) A 2015 report by the WHO
notes that heat waves are an emerging public health problem as climate change worsens, (39) which
further suggests that conditions such as heat stroke and its sequelae may become more common in the
future. This report also points to existing supportive evidence regarding increased mortality and
morbidity during past heat waves in Europe and other regions.(39)
Amatoxin toxicity due to consumption of poisonous mushrooms is a global problem, though difficult to
estimate incidence to do high likelihood of underreporting; while more common in some regions such
as Europe, the literature includes reports of mushroom poisoning in numerous regions around the
world and those with poisoning who develop ALF have a poor prognosis in the absence of significant
supportive care and potentially liver transplantation.(40,41)
ALF caused by excess alcohol intake is another serious condition, with estimated 30 day mortality of
30%.(42) Its exact incidence is unknown, but some have estimated that its incidence in alcoholics may
be up to 20%.(43) Providing global context, a WHO report in 2018 estimated that the prevalence of
heavy episodic drinking was around 18% in 2016 globally, and more common in some areas such as
Eastern Europe and sub-Saharan Africa,(44) suggesting that some regions may be at risk of increased
prevalence of this type of ALF.
Despite the prevalence of a range of conditions precipitating ALF in countries around the world and the
potentially catastrophic nature of ALF for affected child and adults, the EML does not contain any
specific, targeted treatment for this condition, outside of use of NAC specifically for
9
acetaminophen-induced toxicity. The literature indicates growing use of across a range of subtypes
of non-acetaminophen-induced liver failure, with significant off label use and supportive prospective
and retrospective data, described further below and suggesting that this intervention would provide a
valuable addition to the supportive care provided to these patients. Adding this information to the EML
would also provide critical guidance to health workers regarding standard dosing and administration of
NAC as supplemental treatment.
9. Review of benefits: summary of evidence of comparative effectiveness.
Identification of clinical evidence (search strategy, systematic reviews identified, reasons for
selection/exclusion of particular data)
The studies from the literature for this analysis were identified by a trained information scientist
searching the PubMed and Web of Science databases, as well as a broad Google search to identify
unindexed and grey literature. The search terms used were: “acute liver failure”, “acute liver injury”,
“acetylcysteine”, “N-acetylcysteine” and “acetylcysteine”. This search was not date limited; studies were
assessed without restriction by a publication date threshold to ensure inclusiveness. The reference lists
of reviewed articles were also assessed, to identify any studies not found by the initial search and to
better clarify preclinical and mechanistic underpinnings of both the disease and the therapy. No studies
investigating the use of NAC in treatment of ALF with the specified etiologies (selected due to
glutathione involvement, including hepatitis A or B, dengue fever, heat stroke, alcohol poisoning, and
mushroom toxicity) were excluded from this exploration; we further included any studies examining
general non-acetaminophen-induced ALF to complement the evidence pool identified related to the
specified ALF etiologies. Evidence was systemically extracted (see Literature Summary); when
comprehensive systematic reviews and meta-analyses were identified, additional primary evidence was
extracted from other papers to represent 1) data not covered in those reviews/meta-analysis and 2)
nuances of data to complement the data summarized in the systematic reviews. This review also
identified studies evaluating hepatic effects of NAC beyond the selected indications described in the
current application to aid in contextualization; this broader evidence is provided for additional context
in Appendix 2.
Summary of available data (appraisal of quality, outcome measures, summary of results)
Full details of the literature describing each of the subsets of literature described in this section are
included in the evidence tables represented in the Literature Summary section later in this application.
Here, we focus on key characteristics of the primary and secondary literature supporting each of the
ALF subsets proposed in the current application, including the literature describing use of NAC in
general non-acetaminophen-induced ALF as these studies typically represent a number of the narrower
populations we propose.
General non-acetaminophen-induced acute liver failure: Three systematic reviews, published in
2004,(2) 2013,(3) and 2015(4) provide useful insights into the evolution of evidence regarding the use
of NAC in non-acetaminophen-induced ALF. While the two older articles note potential utility of NAC in
this subset of ALF based on data pools comprised primarily of retrospective case reports and series,(2,3)
the 2015 review included four RCTs and concluded significant benefit with use of NAC as compared with
control in terms of transplant-free survival and post-transplantation survival. All three systematic
reviews noted that adverse effects in this population were consistent with those observed in its use in
acetaminophen-induced ALF and that no hepatotoxic effects were seen with the dose used for
acetaminophen toxicity. One additional RCT in non-acetaminophen-induced ALF (n=80) published in
2017, after the 2015 review, also found positive effects of NAC administration; more patients (72.5%)
survived in the NAC group than in the control group (47.5%) (p=0.025) and among those who survived,
10
hospital length of stay was approximately 2.5 days shorter in the NAC-treated group (p=0.002).(45)
Further, a large prospective multisite cohort in the US found increasing use of NAC over time suggesting
significant acceptance of this agent as a clinically attractive off-label use across centers, with almost 70%
of patients with non-acetaminophen-induced ALF receiving this intervention in an 8-year time period
through 2013, further paralleling an increase in survival rates during this time.(46)
Heat stroke associated acute liver failure: In addition to representation of this ALF population in the
general ALF studies described above, we identified 3 case reports suggesting improvement in liver
function and other clinical outcomes associated with use of IV NAC in patients with heat-related
ALF.(47–49) No adverse effects discordant with use of NAC in other indications were identified.
Severe acute alcoholic hepatitis: Severe acute alcoholic hepatitis is somewhat unique among the
causes of ALF, in that it represents an acute event likely embedded within chronic disease; NAC has
been used with success during this acute event, thus we include it here. In addition to representation of
this subgroup of patients in the general ALF studies summarized above, a systematic review in 2015
analyzed the literature regarding use of various therapies in treatment of acute alcoholic hepatitis
requiring hospitalization.(50) This review identified 22 RCTs comprising a total of 2621 patients and
including 5 different interventions. A network meta-analysis of this moderate quality evidence pool
found that the use of corticosteroids alone (relative risk [RR], 0.54; 95% credible interval [CrI], 0.39-
0.73) or in combination with NAC (RR, 0.15; 95% CI, 0.05-0.39), to reduce short-term mortality. No trials
published since the date of this literature review have been identified in the literature.
Mushroom-induced acute liver failure: In addition to representation in the general ALF studies
described above, acute liver injury and failure are a common and severe consequence of mushroom
poisoning. A 2020 systematic review examine the literature on use of NAC in this population, identifying
13 studies including a total of 506 patients.(51) Mortality in patients treated with NAC was 8-11%, liver
transplantation rate was 4.3%. Various laboratory values related to liver function and coagulopathy
improved over 4-7 days after ingestion. Anaphylactoid reactions occurred in 5%. The review concludes
that NAC appears to be safe and beneficial in this type of poisoning.
Acute viral hepatitis: In addition to representation in the ALF studies described above, two small
retrospective case series of NAC use in children with ALF in the context of acute viral hepatitis have been
published, including 40(52) and 12(53) patients respectively. Hepatitis A appeared to be the most
common etiology. Both reports indicate improvement of liver enzymes and coagulation parameters and
satisfactory medication tolerance with use of NAC in the population.
Dengue fever: Given the size of the literature describing use in dengue virus-associated liver injury and
failure, we elected to describe these data separately from the studies of acute viral hepatitis, in which
hepatitis A was most common as the precipitating viral infection. The data collected from various
studies of dengue-infected patients do not include a large, randomized, double-blind, controlled trial.
Given the sporadic and epidemic nature of the disease, such a study would be time-consuming and
costly. We have assembled the existing evidence base on this use, comprising retrospective cohort
studies, case series, and case reports and totaling 43 patients with dengue infection receiving NAC in
addition to usual care. Dengue-related illnesses ranged in severity (but none appeared to be affected by
mild disease). Outcome measures included liver function testing, mortality, measures of morbidity such
as need for transplant, length of stay, and other laboratory measures relevant for dengue fever and its
sequelae. Observed adverse effects were consistent with the broader evidence base on NAC use in
humans, and all patients recovered except 3 patients, with disease level III–IV who already had dengue-
associated ALF prior to treatment, who died. Notably, in one case with dengue associated severe
hepatitis in a 53 year old, prior to NAC treatment, liver enzymes reached peak values of AST 16261 U/L
and ALT 4545 U/L on 4
th
day of admission (7
th
day of illness).(54) Authors note marked improvement
in liver enzyme values, and AST and ALT levels dropped by more than half by 48 hours of treatment. In
a retrospective case series, 13 people with moderate to severe hepatitis received NAC and had hepatic

11
recovery faster than less sick patients who did not receive NAC.(55) Data from case series and case
reports, gradual normalization of liver function tests was noted in 26 other patients (15 adults; 11
infants and children) receiving NAC in moderate to severe dengue illness. (56–67)
PheWAS data: We also reviewed a set of data in which a phenome-wide association study (PheWAS)
analysis was undertaken. PheWAS can identify diseases or conditions (phenotypes) that are associated
with a specific gene/genetic variant.(68) PheWAS leverages existing data from the Exomechip
genotyping platform (~250,000 coding variants across the protein coding region of the genome) and
electronic health records for approximately 35,000 patients. Because the logic of PheWAS can be
extended to predict phenotypic manifestations of pharmacological targeting (such as with NAC) of a
given gene product in humans, we use these methods for drug repurposing.(69) As a glutathione
synthetase (gene: GSS) ‘stimulator’, NAC is hepatoprotective. This is established in its use in
acetaminophen overdose. The phenotypes associated with a missense single nucleotide polymorphism
(SNP) (R418Q) in the GSS gene are risk causing, so in this regard we can say the SNP is behaving like a
glutathione synthetase inhibitor (the opposite of the drug). Thus, the variety of liver phenotypes in the
below analysis (Table 1) strengthen, with human data, that decreased glutathione synthetase is
associated with a broad range of liver injury, as is true in the ALF etiologies represented in the current
application for a new NAC indication on the EML.
Table 1: PheWAS results, GSS variation and liver disease
Note: The SNP in this table appears to be functioning as a glutathione synthetase inhibitor (GSS ↓); thus,
a glutathione synthetase stimulator such as NAC (GSS ↑) is indicated for management of relevant
phenotypes.
rsID (Mutation)
Gene
Phecode
Phenotype
Cases
(n)
Controls
(n)
Odds
ratio
(OR)
P
AFF_11
AFF_12
rs150141794
(Missense_R418Q)
GSS
530.2
Esophageal bleeding
(varices/hemorrhage)
394
18594
5.9300
0.002337
0
5
rs150141794
(Missense_R418Q)
GSS
261.2
Vitamin B-complex
deficiencies
557
21366
4.5910
0.00661
0
5
rs150141794
(Missense_R418Q)
GSS
571.8
Liver abscess and
sequelae of chronic
liver disease
598
22795
4.2490
0.008865
0
5
rs150141794
(Missense_R418Q)
GSS
573.7
Abnormal results of
function study of liver
890
22795
3.4230
0.01187
0
6
rs150141794
(Missense_R418Q)
GSS
571
Chronic liver disease
and cirrhosis
1312
22795
2.7070
0.02196
0
7
rs150141794
(Missense_R418Q)
GSS
571.51
Cirrhosis of liver
without mention of
alcohol
769
22795
3.3010
0.02317
0
5
rs150141794
(Missense_R418Q)
GSS
571.5
Other chronic
nonalcoholic liver
disease
1092
22795
2.7880
0.0282
0
6
rs150141794
(Missense_R418Q)
GSS
573.2
Liver replaced by
transplant
368
22795
4.1420
0.04077
0
3
rs150141794
(Missense_R418Q)
GSS
571.81
Portal hypertension
399
22795
3.8190
0.04958
0
3
Key: GSS glutathione synthetase gene; AFF_11 cases carrying two copies of the variant minor allele; AFF_12 cases carrying
one copy of the variant minor allele

12
Summary of available estimates of comparative effectiveness
With typical comparators in this literature on use of NAC in various types of non-acetaminophen-
induced ALF including either supportive care alone or placebo, the evidence indicates that use of NAC
represents at least an incremental benefit over usual care alone. The literature does not include head-
to-head comparisons with other “active” interventions, precluding a more thorough and quantified
estimate of the comparative effectiveness of NAC. The safety profile reported in this use is consistent
with published adverse effects of NAC use for other indications, suggesting that the risk benefit for this
approach is not weakened by a disparate safety signal.
10. Review of harms and toxicity: summary of evidence of safety.
Estimate of total patient exposure to date:
The WHO EML currently lists N-acetylcysteine as an antidote for the treatment of acetaminophen
overdose.(70) Based on exposure reported in the literature, and given that both oral and IV NAC have
been approved as a first-line therapy for acetaminophen overdose for 40+ years, it is estimated that
hundreds of thousands of patients have been exposed to date (likely many more worldwide).
Description of the adverse effects/reactions when used in non-acetaminophen induced
acute liver failure:
The safety data collected for studies of NAC in non-acetaminophen induced liver failure is captured from
clinical trials, retrospective cohorts, case series, and case reports, comprising data from approximately
2500 patients.(3,4,45–49,51–54,57–59,61,63–65,67,71–81) The adverse effects observed in this
literature are consistent with the broader evidence base on NAC use in humans showing that it is safe
and well tolerated. Investigators report an adverse effect profile observed with use of NAC in non-
acetaminophen induced ALF (general, heat stroke, acute alcoholic hepatitis, mushroom poisoning, acute
viral hepatitis, dengue fever) concordant with the established safety profile of this agent in its use for
acetaminophen induced ALF. Review of adverse effects observed in studies exploring therapeutic use
of NAC in other liver-related conditions and indications (see Appendix 3) also indicates risks similar to
those observed during NAC use for acetaminophen overdose.
Description of the adverse effects/reactions and estimates of their frequency (drawn from
the broader NAC literature on human use)
- Oral administration of NAC is documented to be safe and well-tolerated. The most common side
effects include nausea and vomiting, which is reported to occur in up to 23% of patients(82)
(this may be attributed to its distasteful odor). Oral NAC is rarely associated with more severe
side effects like angioedema.(83,84)
- IV administration of NAC is also usually well-tolerated but is associated with a higher risk of
adverse effects, the most common include:
o Nausea, vomiting – occurs at a frequency of up to 9%(82)
o Anaphylactoid reactions (rash, pruritis, angioedema, bronchospasm) – occurs at a
frequency of 8.2% (out of 6455 treatment courses). 75% of anaphylactoid reactions
were cutaneous(85)
▪ Risk factors for anaphylactoid reactions:
• Females(86) and patients with asthma(87) appear to be at higher risk of
developing the anaphylactoid response and both are associated with a
more severe reaction(85)
13
• Anaphylactoid reactions occur more commonly with lower
acetaminophen levels rather than high levels (this may be because
acetaminophen decreases the histamine released from mast cells and
mononuclear cells, proportionate to the dose ingested). (88)
▪ It is noted that hypersensitivity reactions may be managed by decreasing the
infusion rate or discontinuing the infusion.(83,89)
o Serious adverse reactions and fatalities are rare but have occurred with IV treatment
(these patients also had a history of asthma)(90)
o A tabular summary of the results of systematic reviews of NAC safety when used in non-
dengue indications is included in Appendix 3 .
o While thorough analyses of pharmacovigilance databases (e.g. US FDA FAERS, WHO
Vigibase) are not currently available in the published literature for NAC, the package
inserts for NAC benefit from the long-standing use of this agent for management of
acetaminophen overdose. Post marketing events summarized in these
materials(27,91) include:
▪ Adverse effects identified through post-marketing experience for NAC injection:
rash, urticaria, and pruritus. The frequency of adverse reactions have been
reported to be between 0.2% and 21%, and they most commonly occur during
the initial loading dose of acetylcysteine.
▪ Adverse effects identified through post-marketing experience for oral NAC:
nausea and vomiting, other gastrointestinal symptoms, and rash with or without
fever, and upper GI hemorrhage. (Frequency not reported.)
Summary of available data
- NAC therapies, given via various routes of administration (oral, IV, or inhaled), have been
marketed in the US (and other countries) for over 40 years. Systematic reviews of NAC treatment
for approved and non-approved indications are abundant and suggest that it is a safe and well-
tolerated drug in both pediatric(75,92) and adult populations(83,85,93–96), although particular
attention should be paid to dosing per body weight in pediatric populations to avoid toxicity
related to dosing errors.(97)
- Side effects associated with NAC treatment are typically mild and while nausea and vomiting is
the most common side effect with both IV and oral routes, the rate of nausea and vomiting is
higher with oral NAC. Anaphylactoid reactions are more common with IV NAC and typically
subside upon ceasing treatment. Symptoms characteristic of anaphylactoid reactions include
flushing, pruritus, and rash and can also include angioedema, bronchospasm, and hypotension.
Severe adverse reactions and fatalities are rare.(82)
- NAC drugs are available internationally.(89,98) For example regarding availability of various
formulations, there are 7 drugs currently on the market in the US given via IV route of
administration, 4 given orally (effervescent tablet or oral solution), and 3 given via inhaled
solution.(99)
- Safety information from package inserts for example NAC therapies is presented below (Table
2; adverse events for various routes of NAC administration including oral, IV, and inhaled
routes).
- Several randomized control trials of NAC for acetaminophen overdose have also been reported.
One relatively small randomized control trial (n = 50) randomized patients with hepatic failure
after acetaminophen overdose to either IV NAC in addition to standard liver care or standard
liver care alone.(100) The NAC regimen in this study included: 150 mg/kg body weight in 200
ml 5% dextrose over 15 minutes, followed by 50 mg/kg in 500 ml 5% dextrose over four hours,
then 100 mg/kg in 1 L over 16 hour. The final infusion rate was continued until recovery from

14
encephalopathy or death. The rate of survival was higher in patients receiving NAC. No adverse
side effects were reported in this study.
- Another larger trial(101) that randomized 223 patients to different 150 mg/kg N-acetylcysteine
loading infusion rates (15 minutes or 60 minutes) reported adverse event rates of 75% and 61%
for the 15 minute and 60-minute arms, respectively. Anaphylactoid reactions were the most
reported adverse reactions in both arms, occurring in 18% in the 15-minute arm and 15% in the
60-minute arm. Two patients (one in each arm) experienced a severe anaphylactoid reaction
and were withdrawn from the study. Nausea and vomiting, classified within the broader GI
disorders category in study analyses, were experienced by 13% of patients. The difference
between the drug-related adverse events was not statistically significant and no deaths were
reported
- The remaining body of clinical trial literature is comprised of prospective, non-randomized,
observational trials.(83,85,93,94) Nevertheless, the data from these studies support the RCTs
above showing that both oral and IV NAC are safe and well tolerated. Case reports have also
described other rarer features of anaphylactoid reactions like ECG abnormalities(102), status
epilepticus(103), and a serum sickness-like illness(104), however these are not commonly
reported in larger trials.
Table 2: Adverse event summary for various NAC formulations
Drug (route)
Population
Indication
Adverse event (and frequency, if
reported)
Reference
CETYLEV (oral
effervescent tablet)
Adults and
children, though
pediatric
approval is not
based on
adequate or
well-controlled
studies
Acetaminophen
overdose
- Allergic reaction
- Nausea and vomiting (up to 30% of
patients)
- Rash (with or without fever)
- GI problems
- May aggravate vomiting as a
symptom of acetaminophen
overdose
- May aggravate vomiting and
increase risk of upper GI
hemorrhage in at risk patients
(those with esophageal varices,
peptic ulcers)
- Hypersensitivity reactions,
including generalized urticaria
U.S package
insert(91)
ACETADOTE (IV)
Adults
Acetaminophen
overdose
- Pruritis (4.3%)
- Urticaria/facial flushing (6.1%)
- Respiratory symptoms (1.9%)
- Edema (1.6%)
U.S package
insert(27)
Children
Acetaminophen
overdose
- Urticaria/facial flushing (7.6%)
- Pruritis (4.1%)
- Respiratory symptoms (2.2%)
- Edema (1.2%)
Geriatric
Acetaminophen
overdose
- Clinical studies do not provide
sufficient number of geriatric
subjects to determine whether the
elderly respond differently
- There are not adequate and well-
controlled studies in pregnant
women, but limited case reports do
not include any adverse maternal,
fetal, or neonatal outcomes
Acetylcysteine 200
mg/mL injection (IV)
Adults and
children
Acetaminophen
overdose
- The most common AEs are nausea,
vomiting, flushing, and skin rash
- Less commonly, more serious
anaphylactoid reactions have been
reported (angioedema,
bronchospasm, hypotension,
tachycardia, or hypertension)
- AEs usually occur between 15 and
60 min after start of infusion (many
Europe/UK
package
insert(105)

15
symptoms are relieved by ceasing
infusion)
- Other reported AEs include:
infection site reaction, pruritus,
cough, chest tightness or pain, puffy
eyes, sweating, malaise, raised
temperature, vasodilation, blurred
vision, bradycardia, facial or eye
pain, syncope, acidosis,
thrombocytopenia, respiratory or
cardiac arrest, stridor, anxiety,
extravasation, arthropathy,
arthralgia, deterioration of liver
function, generalized seizure,
cyanosis, lowered blood urea
- Fatalities are very rare
- Hypokalemia and ECG changes have
been noted in patients with
acetaminophen overdose,
monitoring of plasma potassium
concentration is recommended
Acetylcysteine
solution, USP
(inhaled)
Adults and
children
Chronic or acute
bronchopulmonary
disease, pulmonary
complications of
cystic fibrosis, and
other conditions
associated with
abnormal, viscid, or
inspissated mucous
secretions
- Stomatitis, nausea, vomiting, fever,
rhinorrhea, drowsiness,
clamminess, chest tightness and
bronchoconstriction.
- Clinically overt bronchospasm
occurs infrequently and
unpredictably even in patients with
asthmatic bronchitis or bronchitis
complicating bronchial asthma
Package
insert(106)
Acetaminophen
overdose
- Oral administration of the large
doses needed to treat
acetaminophen overdose may
result in nausea, vomiting, and
other GI disorders
- Rash, with or without fever, has
been reported but rarely
Parvolex (200 mg/ml
concentrate solution
for infusion)
Adults and
children
Acetaminophen
overdose
- Swelling of the face, lips or tongue
- Wheezing, difficulty breathing
- Irritation at the injection site
- Skin rash, itching
- Flushing
- Low blood pressure resulting in
dizziness
- Rapid heartbeat or increased blood
pressure (rarely)
- Other rare side effects include
coughing, noisy breathing,
respiratory arrest, chest tightness
or pain, puffy eyes, blurred vision,
sweating, raised temperature, liver
problems, slow heartbeat, fainting
or collapsing, reduction in blood
platelets
Package
insert(107)
Identification of variation in safety that may relate to health systems and patient factors
- Studies using IV NAC for acetaminophen overdose have shown that females(86) and those with
a history of asthma or atrophy(87) are particularly susceptible to anaphylactoid reactions.
- The package insert for CETYLEV (oral, effervescent NAC tablets) states that it may aggravate
vomiting as a symptom of acetaminophen overdose and may aggravate vomiting and increase
risk of upper GI hemorrhage in at risk patients (those with esophageal varices, peptic
ulcers).(91)

16
- Reduced clearance of NAC in seven patients affected by chronic liver disease as compared with
six healthy controls, suggesting that it is possible that cirrhotic patients may be at increased risk
of hypersensitivity reactions.(26)
- As NAC is a nitrogenous substance, a theoretical risk of hepatic encephalopathy (HE) is noted in
some NAC package inserts, which further note that there is no clinical data suggesting that
acetylcysteine influences on hepatic failure.
11. Summary of available data on comparative cost and cost-effectiveness
of the medicine.
NAC is already on the EML, with a widespread availability in most countries of the world at very low
cost. The current application is not a request to add a medication for which pricing would be needed, as
there would be no change to the existing pricing data expected from adding this new use of NAC.
Considering one of the more extreme outcomes of ALF, liver transplantation has varied costs and
availability in different settings; in the United States, for example, a recent report noted that the average
liver transplant was billed at over $800,000 per patient(108); while it is likely that the US is on the upper
end of the global spectrum of costs for this procedure,(109) the resources required for transplant and
follow-up are likely intensive in most settings, compounded further by the limited availability of organs
for transplant. The estimated cost for NAC is US$70. Given the extremely low NAC price per dose and
the potential for averting significant downstream outcomes such as need for liver transplantation, its
use would have substantial cost effectiveness.
12. Summary of regulatory status and market availability of the medicine.
NAC is approved by many health authorities for prevention of liver injury in acetaminophen overdose
or as a mucolytic. To our knowledge, no health authority currently has NAC formally listed for a liver
indication outside of acetaminophen overdose despite it being used in this setting. The lack of financial
incentives for the pharma manufacturing industry to pursue new regulatory approvals for a medication
that is no longer proprietary likely prevents this from happening. Examples of NAC approval for use in
various countries are as follows:
Regulatory Agency
Indication
US Food and Drug Administration (FDA)
- To prevent or lessen liver injury after
acetaminophen overdose
- Mucolytic in patients with cystic fibrosis
(or other conditions associated with
abnormal or viscid mucous secretions)
European Medicines Agency (EMA)
- To prevent or lessen liver injury after
acetaminophen overdose
- Mucolytic in patients with cystic fibrosis
(or other conditions associated with
abnormal or viscid mucous secretions)
Australian Government, Department of Health,
Therapeutic Goods Administration
- To prevent or lessen liver injury after
acetaminophen overdose
- Mucolytic in patients with cystic fibrosis
(or other conditions associated with
abnormal or viscid mucous secretions)

17
Japanese Pharmaceuticals and Medical Devices
Agency
- To prevent or lessen liver injury after
acetaminophen overdose
- Mucolytic in patients with cystic fibrosis
(or other conditions associated with
abnormal or viscid mucous secretions)
Health Canada
- To prevent or lessen liver injury after
acetaminophen overdose
- Mucolytic in patients with cystic fibrosis
(or other conditions associated with
abnormal or viscid mucous secretions)
Further, there is widespread market availability of NAC and multiple generic manufacturers including
Fresenius Kabi, Auro Medics Pharma, Cadila Healthcare, Zydus Pharmaceuticals, Roxane Laboratories
Inc., Sagent Pharmaceuticals, and Pfizer, among many others in various countries. Given that NAC is in
widespread use globally as an acetaminophen overdose antidote and as a mucolytic, it is anticipated that
the currently proposed expanded use for this agent would leverage the existing supply chains
established in various regions.
13. Availability of pharmacopeial standards (British Pharmacopoeia,
International Pharmacopoeia, United States Pharmacopoeia, European
Pharmacopeia). Summary of available data on comparative cost and cost-
effectiveness of the medicine.
Acetylcysteine is included in several pharmacopeial standards, including the British Pharmacopoeia; the
United States Pharmacopoeia; and the European Pharmacopoeia.

18
Literature summaries: non-acetaminophen acute liver failure, organized by precipitating
exposure/condition
LITERATURE SUMMARY: Evidence describing use of NAC in general non-acetaminophen-induced acute liver injury
First author, year
country
Design
Condition
Sample
size
NAC dose, frequency,
duration, route of
administration; comparator
Findings
Outcome
Hu, 2015(4)
Meta-analysis
Non-
acetaminophen
induced acute liver
failure
4 clinical
trials
(total n
331 NAC,
285
control)
NAC as administered in
original clinical trials,
compared to control arm
No statistical difference was identified
between NAC group and control group for
overall survival [236/331 (71%) vs
191/285 (67%); 95% CI 1.16 (0.81-1.67);
P=0.42].
There were significant differences between
NAC group and control group regarding the
survival with native liver [112/273 (41%)
vs 68/226 (30%); 95% CI 1.61 (1.11-2.34);
P=0.01] and post-transplantation survival
[78/91 (85.7%) vs 50/70 (71.4%); 95% CI
2.44 (1.11-5.37); P=0.03].
Side effects included nausea, vomiting, and
diarrhea or constipation; rarer effects
included rashes, fever, headache,
drowsiness, low blood pressure, and
elevated serum transaminase levels in a
patient with cystic fibrosis.
No hepatotoxic effects observed at the dose
used for acetaminophen toxicity.
Positive
effect
Sales, 2013(3)
Systematic
review
Non-
acetaminophen
induced acute liver
failure
11
articles
included
(8 case
reports, 2
retrospec
tive trials,
1 RCT)
NAC as administered in
original report
The 2 retrospective studies suggested
survival benefit in adults and children; RCT
suggested benefit in terms of transplant-
free survival.
Oral and IV NAC well tolerated.
Authors concluded marginal benefit of NAC
Suggests
positive effect
Sklar 2004(2)
Systematic
review
Non-
acetaminophen
7 studies
NAC as administered in
original report
Investigators commented: “All of the
studies found were small and do not
provide conclusive evidence that
Suggests
positive effect
related to
19
induced acute liver
failure
acetylcysteine benefits this subgroup of
patients. Microvascular regional benefits
were seen, but clinical outcomes have not
been studied.”
microvascular
regional
benefit
Nabi, 2017(45)
Randomized
study
Non-
acetaminophen-
induced liver
failure (etiology
included
undetermined,
hepatitis E, other
drugs and toxins,
Wilson disease,
autoimmune
disease, CMV, HSV)
80
IV NAC initial loading dose of
150 mg/kg over 1 hour,
followed by 12.5 mg/kg/h for
4 hours and continuous
infusion of 6.25 mg/kg/h for
remaining 67 hours.
Control patients received 5%
dextrose infusion for 72 hours.
Incidence of renal failure was not
significantly different between the two
groups. Mannitol for increased ICP was
used more often in the control group as
compared with the NAC group (92.5 vs
75%, p=0.037). Among the patients who
survived, mean hospital length of stay was
shorter in the NAC group (8.241 ± 2.115 vs
10.737 ± 3.106, p=0.002).
A total of 32 of 80 (40%) patients died with
ALF complications; 11 (27.5%) patients
belonged to the NAC group and 21 (52.5%)
patients to the control group (chi‑square =
5.208; P = 0.023) and the mean time to
death from diagnosis was 9.3 days.
More patients (72.5%) survived in the NAC
group than in the control group (47.5%)
(p=0.025) Stratification by etiology
suggested that patients with drug‑induced
ALF showed improved outcomes..
No adverse effects attributable to NAC were
observed.
Positive
effect
Lee, 2009(71),
Stravitz,
2013(73)and Singh,
2013(72)
USA
RCT
Non-
acetaminophen-
induced liver
failure
Majority fell into 4
etiologies: drug-
induced liver
injury (n=45),
autoimmune
hepatitis (n=26),
hepatitis B (n=37)
and
indeterminate (n=
41)
173
NAC infusion in 5% dextrose:
an initial loading dose of 150
mg/kg/h of NAC over 1 hour,
followed by 12.5 mg/kg/h for
4 hours, then continuous
infusions of 6.25 mg/kg NAC
for the remaining 67 hours (3
days total) (81 assigned, 48
completed 72h trial, 33
received less than full
treatment because of death,
withdrawal of support,
transplantation, or side effects
of drugs (4 thought to be due
to NAC specifically))
Transplant-free survival was significantly
better for NAC patients (40%) than for
those given placebo (27%; 1-sided P =
.043).
The transplantation rate was lower in the
NAC group but was not significantly
different between groups (32% vs 45%; P =
.093).
Adverse effects: Nausea and vomiting
occurred significantly more frequently in
the NAC group (14% vs 4%; P=0.031).
Positive
effect

20
Subjects in the placebo group
received infusion of 5%
dextrose only (92 assigned, 58
completed 72h trial, 34
received less than full
treatment)
Treatment group and day of study in
models including bilirubin or ALT were
predictors of transplantation or death
(maximum p < 0.03). Those patients with
early coma grade who were treated with
NAC showed significant improvement in
bilirubin and ALT levels when compared to
the other three groups (maximum p < 0.02
for NAC 1-2 vs. the 3 other treatments)
when predicting death or transplantation.
Treatment group, day of study, and
bilirubin were predictors of transplantation
(maximum p < 0.03) in ALF patients.
Stepwise multivariate logistic regression
analysis identified only NAC administration
and lower IL-17 concentrations as
independent predictors of transplant-free
survival. In patients with detectable IL-17
concentrations on admission, 78% of those
who received NAC vs. 44% of those who
received placebo had undetectable levels by
day 3-5 (P = 0.042), and the mean decrease
in IL-17 concentrations between admission
and late samples was significantly greater
in patients who received NAC vs. placebo (P
= 0.045).
Squires, 2013(110)
USA
Double-blind,
placebo-
controlled RCT
Pediatric acute
liver failure not
believed to be
caused by
acetaminophen
(discharge
diagnoses included
autoimmune,
infection,
metabolic
disorders, and
other conditions; 1
patient had
acetaminophen
overdose and
approximately
184
150 mg/kg/d NAC infusion in
5% dextrose infused over 24
hours for up to 7 consecutive
days (92 subjects)
92 received placebo (dextrose
and water alone)
The 1-year survival did not differ
significantly (p=0.19) between the NAC
(73%) and placebo (82%) treatment
groups.
*The 1-year transplant-free survival was
significantly lower (p=0.03) in those who
received NAC (35%) than those who
received placebo (53%).
There were no significant differences
between treatment arms for hospital or ICU
length of stay, organ systems failing, or
highest recorded grade of HE.
Metabolic disease was more common in the
NAC arm (13 NAC vs 5 placebo) with
Reduced
efficacy?

21
60% had unknown
cause)
Wilson disease (7 NAC vs 3 placebo) being
more common in the NAC arm than the
placebo arm.
Darweesh 2017(74)
Egypt
Prospective
and
retrospective
observational
study
Non-
acetaminophen-
induced liver acute
liver failure
155
IV NAC 150 mg/kg in 100 ml
dextrose 5% over 30 min, then
70 mg/kg in 500 ml dextrose
5% over 4 hr., then 70 mg/kg
in 500 ml dextrose 5% over 16
hr., then continuous infusion
over 24 hr. of 150 mg/kg in
500 ml dextrose 5% until up to
two consecutive normalized
INRs were obtained.
Control group included those
who did not receive NAC
The incidence of transplant-free survival
was 96.4% (n=82) in the NAC-treated
group (p<0.01 compared with control
group); among the 3 remaining patients, 2
received a liver transplant and 1 died.
These 3 patients did not receive the full
dose of NAC, two due to a severe allergic
reaction to NAC (both were transplanted)
In the control group, 17 (23.3%) recovered;
among the remaining 53 patients, 37
(53.3%) received a liver transplant and 16
(23.3%) died.
NAC treated patients had significantly
shorter hospital stays (p<0.001), less
encephalopathy (p=0.02), and less bleeding
(p<0.01) as compared with control patients.
Control patients had higher incidence of
ICU admission (p=0.01) and increased
incidence of abnormal creatine and
electrolytes (p=0.002 and p<0.01,
respectively).
Bilirubin was significantly increased among
controls (p=0.02); AST and INR were
significantly increased among NAC-treated
patients (p<0.001 for both). ALT was not
significantly different between the groups.
Positive
effect

22
Adverse events attributed to NAC included
prolonged cholestasis in 82; bilirubin
showed a steady but slow decrease over 2-3
months; patients not treated with NAC did
not develop this sign. Fever and allergic
reaction were observed in 3 patients and
dyspepsia in 11 patients. No bronchospasm
was observed.
Reuben, 2016(46)
US
Prospective
observational
Acute liver failure
of all causes except
previous liver
transplant; ~46%
acetaminophen
toxicity, the rest
due to
heterogeneous
causes
2070
NAC protocol varied from site
to site; not detailed
Two time periods, 1998-2005, 2006-2013
Use of NAC increased in the 2
nd
time period
(69.3% vs 48.9% in the first time period,
p<0.001) in patients with ALF not due to
acetaminophen toxicity
Overall survival and transplant free
survival increased during the 16 year
period
Other changes in the 2
nd
vs. 1
st
period
included reduced RBC and plasma infusion,
mechanical ventilation, and use of
vasopressors.
Suggests
positive effect
+ significant
off label use in
the US
Mumtaz, 2009(76)
Pakistan
Prospective
non-blinded
study with
historical
controls
Acute liver failure
not caused by
acetaminophen
(majority were due
to hepatitis E or B
virus, but some
due to
antituberculosis
treatment)
91
Oral NAC dose of 140 mg/kg
followed by 70 mg/kg, for a
total of 17 doses 4 hours apart
within 6 hours of admission
(47 subjects prospectively
enrolled)
44 subjects received standard
care only (historical controls
from hospital database)
A total of 34 (37.36%) patients survived; 22
(47%) in group 1 (NAC group) and 12
(27%) in group 2 (controls) (P = 0.05),
indicating NAC causes a significant
reduction in mortality. (no liver specific
outcome measures)
On multivariable regression analysis,
patients not given NAC (odds ratio
[OR] = 10.3, 95% confidence interval
[CI] = 1.6–65.7), along with age older than
40 years, patients requiring mechanical
ventilation, and interval between jaundice
and hepatic encephalopathy were
independent predictors of mortality.
Presumed
positive effect

23
Kortsalioudaki,
2008(75)
UK
Retrospective
review
Pediatric acute
liver failure not
believed to be
caused by liver
failure
170
Continuous IV infusion NAC
100 mg/kg/24 hours until INR
normalization, death, or liver
transplant. Median duration 5
days (range 1-77)
Compared with historical
group receiving supportive
care without NAC
Length of hospital stay, length of ICU stay,
and incidence of death without liver
transplant were not significantly different
between the two groups. The 10 year
actuarial survival was 50% in the
supportive care group and 75% in the NAC
treated group (n=0.009). Survival with
native liver was observed in 13 (22%) of
the supportive care group and 48 (43%) of
the NAC-treated group. Death after
transplantation occurred in 15 (39%) of the
supportive care group as compared with 8
(16%) of the NAC treated group (p=0.02).
Among NAC-treated patients, side effects
were noted in 8 (10.8%), including rash
(n=3) resolving with no treatment;
bradycardia (n=2) or tachycardia (n=1)
attributed to underlying disease; dizziness
and peripheral edema (n=1) with NAC
tolerated at lower dose; and bronchospasm
and florid maculopapular rash attributed as
an allergic reaction to NAC requiring
discontinuation.
Presumed
positive effect
Ben-Ari, 2000(77)
Retrospective
observational
Acute liver failure
not caused by
acetaminophen
7
NAC administered at
presentation
Clinically, 3 patients who initially had grade
O/II encephalopathy, did not progress, and
have fully recovered. The mean peak
prothrombin time, serum factor V,
aspartate aminotransferase and alanine
aminotransferase levels, all significantly
improved. Four patients (57%) have
recovered fully (1 patient, although fully
recovered, died later from an unrelated
cause). Two patients required orthotopic
liver transplantation and 1 patient died. N-
acetylcysteine administration may have
prevented progression to grade III/IV
encephalopathy and improved serum
coagulation factors.
Presumed
positive effect
Harrison 1991(78)
UK
Case series
Acute liver failure
12 due to
acetamin
ophen
and 8 due
NAC was given in a dose of 150
mg per kilogram of body
weight in 250 ml of 5 percent
dextrose over a period of 15
minutes and then in a dose of
Positive effects in patients with non
acetaminophen induced liver failure were
similar to those observed in the
acetaminophen group.
Presumed
positive effect

24
to other
causes
50 mg per kilogram in 500 ml
of 5 percent dextrose over a
period of 4 hours
LITERATURE SUMMARY: Evidence describing use of NAC in heatstroke-associated acute liver injury
First author, year
country
Design
Condition
Sample
size
NAC dose, frequency,
duration, route of
administration; comparator
Findings
Outcome
Monzon 2020(47)
US
Case report
Heatstroke-
associated ALF
1
NAC IV initiated hospital day 2
for ALI. NAC was infused at
15,000 mg IV over one hour,
followed by 5000 mg IV over
four hours, then 10,000 mg IV
over 16 h without continuation
of therapy.
24-year-old unresponsive male without
significant past medical history presented
to the emergency department with heat
stroke; his initial temperature was 107.4 °F.
During his hospital course, he developed
ALI with significant elevation in aspartate
aminotransferase, alanine
aminotransferase, and total bilirubin. These
laboratory findings peaked by hospital day
two, but improved prior to discharge on
hospital day five and throughout his follow
up clinic visits. His treatment course
included cooling measures, supportive care,
supplemental oxygen and airway
management, seizure control, and
intravenous NAC therapy.
Suggest
positive effect
Will 2019(49)
US
Case report
Heatstroke
associated ALF
1
Starting hospital day 3, loading
dose of NAC at 150 mg/kg was
given over one hour. NAC
therapy was continued at a
dose of 12.5 mg/kg/hr for four
hours with steady clinical
improvement. Following
stabilization, he was
transferred back to the
military treatment facility,
where he completed a 72-hour
total course of NAC continued
at 6.25 mg/kg/hr.
27-year-old basic combat trainee presented
with altered mental status, renal
insufficiency, rhabdomyolysis, and a core
temp of 107.9 °F after collapsing during a
run, leading to the diagnosis of heat stroke.
While the patient's azotemia and creatinine
kinase levels rapidly improved with
aggressive intravenous hydration,
transaminases continued to increase to
nearly 155 times the upper limit of normal.
Further laboratory evaluation revealed
coagulopathy and thrombocytopenia
suggestive of acute liver failure (ALF).
Liver function improved on NAC; patient
discharged after 3 days of NAC and
laboratory values returned to normal by 8
weeks.
Suggests
positive effect
25
Aquilina 2018(48)
Malta
Case report
Heatstroke
associated ALF
1
NAC dose not reported;
initiated day 6 and continued
until day 29
31 year old collapsed during a race, had
ALF at admission and liver function
continued to deteriorate. Liver transplant
considered
NAC discontinued at day 29 due to
improvement in liver function; discharged
on day 31.
Suggests
positive effect
LITERATURE SUMMARY: Evidence describing use of NAC in alcohol poisoning-associated acute liver injury
First author, year
country
Design
Condition
Sample
size
NAC dose, frequency,
duration, route of
administration; comparator
Findings
Outcome
Singh 2015(50)
Systematic
review and
meta analysis
Severe acute
alcoholic hepatitis
22 RCTs,
2621
patients,
5
interventi
ons
NAC as used in original reports
in a direct meta-analysis, only
corticosteroids decreased risk of short-
term mortality. In a network meta-analysis,
moderate quality evidence supported the
use of corticosteroids alone (relative risk
[RR], 0.54; 95% credible interval [CrI],
0.39-0.73) or in combination with
pentoxifylline (RR, 0.53; 95% CrI, 0.36-
0.78) or NAC (RR, 0.15; 95% CI, 0.05-0.39),
to reduce short-term mortality; low quality
evidence showed that pentoxifylline also
decreased short-term mortality (RR, 0.70;
95% CrI, 0.50-0.97). The addition of NAC,
but not pentoxifylline, to corticosteroids
may be superior to corticosteroids alone for
reducing short-term mortality.
Positive
effect with
corticosteroi
ds
LITERATURE SUMMARY: Evidence describing use of NAC in mushroom toxin-induced acute liver injury
First author, year
country
Design
Condition
Sample
size
NAC dose, frequency,
duration, route of
administration; comparator
Findings
Outcome
Liu 2020(51)
Systematic
review
Mushroom
poisoning
13
studies,
506
patients
Not detailed; all studies
included NAC intervention
The mortality rate (including liver
transplant patients) of amatoxin-poisoning
patients with NAC treatment was 11%
(57/506), and a the mortality rate
(excluding transplant patients) 7.9%
(40/506) and a liver transplantation rate of
Positive
effect

26
4.3% (22/506). Transaminase
concentrations generally peaked around 3
days after ingestion, prothrombin
time/International Normalized Ratio
(PT/INR) generally worsened during the
first 3-4 days after ingestion before
returning to normal four to 7 days after
ingestion, and Factor V levels normalized in
about 4-5 days after ingestion in patients
treated with NAC. Renal failure was
reported in 3% (3/101) and acute kidney
injury was reported in 19% (5/27).
Gastrointestinal bleeding occurred in 21%
(15/71). Anaphylactoid reactions were the
principle adverse reaction to NAC
treatment in amatoxin-poisoning patients
with an incidence of 5% (4/73).
Authors concluded that NAC appears to be
beneficial and safe.
Karvellas 2016(79)
North America
Registry cohort
Mushroom-
induced ALF and
ALI
18, 13
with ALF
Not detailed
N-acetylcysteine used in nearly all patients
88% of nonspontaneous survivors and 80%
of spontaneous survivors.
No efficacy
inferences
possible;
significant off
label use.
Vanooteghem
2014(80)
Belgium
Case series
Mushroom-
induced ALF
4
Not detailed
All patients survived without need for liver
transplant.
Suggests
positive effect
Montaninia
1999(81)
Italy
Case series
Mushroom-
induced ALF
11
Not detailed, notes “high dose”
All patients survived, 1 with preceding liver
disease required liver transplant
Suggests
positive effect
LITERATURE SUMMARY: Evidence describing use of NAC in virus-associated acute liver injury (hepatitis A, hepatitis B,
dengue fever)
First author, year
country
Design
Condition
Sample
size
NAC dose, frequency,
duration, route of
administration; comparator
Findings
Outcome
Saleem 2015(52)
Pakistan
Case series
Children age 1
month to 16 years
with ALF and acute
viral hepatitis
40
Not detailed
There was significant statistical difference
in liver enzymes and prothrombin time on
admission comparing at discharge in
Suggests
positive
effect

27
children received NAC (p < 0.001). Fifteen
(38%) children died.
Sotelo 2009(53)
Mexico
Case series
Children with
acute viral
hepatitis and acute
hepatic failure
12 (10
with
hepatitis
A)
All received oral NAC, six
patients for a week and the
remaining six for 9-36 days.
Treatment was not ceased
until patients showed clinical
and laboratory improvement.
Significant improvement in liver function
and coagulation parameters compared with
initial values. Investigators note good
tolerance to medication and satisfactory
clinical course
Suggests
positive
effect
Arboviral infections
Paramasivam
2013(55)
Malaysia
Retrospective
cohort
Dengue fever with
moderate or
severe hepatitis
13 of 85
treated
with NAC
NAC administered over 1 hour
followed by maintenance until
AST < 300 mmol/L (NAC dose
not detailed)
Mean (SD) duration of NAC
administration was 2.4 (2.9)
days.
Comparison group: those not
treated with NAC
7 patients had moderate hepatitis (AST
300-9999 mmol/l) and 6 had severe
hepatitis (AST>1000 mmol/l).
Time to ALT reduction to < 300 mmol/l was
3.8 days in patients receiving NAC
compared with those who did not receive
NAC (4.7 days, p=0.003). Length of hospital
stay was significantly longer in those
receiving NAC vs. those not receiving NAC
(6.0 vs. 4.7 days, p=0.009). Authors note
that NAC was given to patients who were
more ill.
Positive
effect
Tan 2013(57)
Malaysia
Retrospective
case series
Severe dengue
fever by WHO
2009
classifications
7 of 8
treated
with NAC
IV NAC infusion 100-150
mg/kg/day for a minimum of 5
days
The maximum grade of hepatic
encephalopathy was grade III in five
patients and grade II in three patients, all
occurring within one to four days of
admission.
All patients discharged well after a median
of 13.5 (5, 35) hospital days; all seven
patients with longer term follow up had
normalization of ALT.
No adverse effects of NAC observed
Presumed
positive
effect
Kumarasena,
2010(58)
Sri Lanka
Retrospective
case series
Dengue-associate
acute liver failure
in the setting of
severe disease (six
patients had
dengue shock
syndrome)
8
Intravenous NAC at 150 mg/kg
loading dose over 15 min
followed by 12.5 mg/kg/h for
4 hours, then 6.25 mg/kg/h for
up to 72 hours
All received other supportive
management
All five patients with hepatic
encephalopathy coma grades I–II recovered
completely and were well at follow-up after
at least 2 months, whereas the three
patients with coma grades III–IV died.
No patients had adverse effects attributable
to NAC.
Presumed
positive
effect

28
Senanayake
2013(59)
Sri Lanka
Retrospective
case series
Dengue-associated
acute liver failure
(pediatric; age 6
months – 12 years)
7
IV NAC 100 mg/kg over 24
hours, continued up to 72
hours in patients with hepatic
encephalopathy at the end of
the first dose
Following the first dose of NAC (100 mg/kg
over 24 hours) four patients showed rapid
clinical improvement of encephalopathy.
Remaining three responded after
second (2) and third (1) doses. In all seven
patients, biochemical profiles showed
improvement from first dose onwards. No
side effects of NAC were noted. All seven
made complete recovery without residual
hepatic or neuro-developmental damage.
Presumed
positive
effect
Kularatne, 2018(60)
Sri Lanka
Retrospective
case series
Dengue-associated
acute liver failure
in adults (age 22
and age 39)
2 of 10
received
NAC
IV NAC (in one case, at 150
mg/hour, duration not noted;
NAC regimen details not
provided for 2
nd
patient)
Case 1: discharged at day 12 with near
normal liver transaminases; follow-up at 21
days indicated normal liver biochemistry
Case 2: symptoms resolved within the next
few days
Presumed
positive
effect
Tan 2016(61)
Singapore
Retrospective
case series
Dengue-associated
liver failure in
pediatrics (age 5
months to 6 years)
2 of 4
received
NAC
IV NAC (100 mg/kg/day) for 1
week
All patients recovered fully. No adverse
effects of NAC observed.
Presumed
positive
effect
Lewis 2019(62)
US
Case report
Dengue shock
syndrome (age 23)
1
NAC (details not given)
Exchange student arrived from India 3 days
before hospital admission, treated with
platelets, NAC, and other supportive care.
Progressed to acute liver failure including
hepatic encephalopathy and was listed for
liver transplant. 24 hours after listing, liver
tests began to improve and mental status
normalized over the next 48 hours, along
with resolution of fever and tachycardia.
Patient was delisted.
Presumed
positive
effect
Dalugama 2018(56)
Sri Lanka
Case report
Dengue fever with
acute liver failure
and acute kidney
injury (age 43)
1
IV NAC 100 mg/hour for five
days
Transaminases gradually declined, lactate
normalized, serum creatinine reduced and
normalized, urine output gradually
increased and CVVHD was terminated after
138 hours of dialysis. Discharged on 9
th
day
after admission.
Presumed
positive
effect
Dalugama,
2017(63)
Sri Lanka
Case report
Dengue fever
(age 53)
1
Intravenous NAC at a rate of
100 mg/hour (in addition to
packed cells transfusion) until
discharge (day 5)
Gradual reduction in AST and ALT,
prothrombin time and activated partial
thromboplastin time normalized. On day 5
of admission AST and ALT were less than
200 U/L and NAC infusion was terminated
and patient discharged.
Patient was in good in health with normal
liver enzymes, clotting profile, and albumin
Presumed
positive
effect

29
at follow up visits on day 7 and 2 weeks
later.
Habaragamuwa
2014(54)
Sri Lanka
Case report
Dengue associated
severe hepatitis
(age 54)
1
Intravenous NAC at 100
mg/kg/day infusion for 5 days
Prior to NAC treatment, liver enzymes
reached peak values of AST 16261 U/L and
ALT 4545 U/L, prothrombin
time/international normalized ratio
(PT/INR) 1.7, and total bilirubin 5.9 mg/dl
on 4
th
day of admission (7
th
day of illness).
NAC administration produced marked
improvement in liver enzyme values, and
AST and ALT levels dropped by more than
half by 48 hours of treatment. On the 9
th
day of admission liver function revealed
AST 300 U/L, ALT 223 U/L and PT/INR 1.2.
During follow up visit at 2 weeks after
discharge, patient had normal liver profile
and no evidence of chronic liver disease.
Presumed
positive
effect
Manoj 2014(67)
Sri Lanka
Case report
Dengue
hemorrhagic fever
with acute liver
failure and massive
bleeding (age 37)
1
IV NAC for 72 hours (dose not
detailed) along with
recombinant factor VIIa and
other supportive therapies
Patient was admitted after 3 days of illness
and moved to ICU after developing agitation
and drowsiness 36 hours after admission,
with intubation around day 5-6 of illness.
Transaminases showed a marked rise (AST
12500 U/L and ALT: 2700 U/L, bilirubin
1.8) in seventh day of illness; massive
hematemesis on day 8 and CVP rise at 9
th
day required furosemide. Transaminases
gradually declined over next several days.
Patient was extubated after 7 days of
ventilatory support and discharged after a
total hospital stay of 18 days (discharge
AST 96 U/L, ALT 109 U/L, bilirubin 16.8
mg/dL).
Presumed
positive
effect
Abeysekera
2012(64)
Sri Lanka
Case report
Dengue fever with
hepatic
encephalopathy
(age 52)
1
IV NAC initiated on day 6 of
fever, just after admission, 150
mg/kg in 100 ml normal saline
over 1 hr., 50 mg/kg in 500 ml
normal saline over 24 hrs. for
3 days
Patient had complete recovery and was
discharged home 10 days after admission
with all parameters returning to normal
levels.
Presumed
positive
effect
Lim 2011(65)
Singapore
Case report
Dengue fever
(pediatric age 6)
1
Intravenous NAC at 100
mg/kg/day as a continuous
infusion over 24h for 6 days
Rapid decrease in liver transaminases: AST
dropped to 5991 U/l and ALT 1789 U/l
after 3 days; AST to 1044 U/l and ALT to
635 U/l, respectively, after 6 days.
Presumed
positive
effect

30
Coagulation profile normalized: PT
improved to 18.0 s and INR 1.54 after 6
days.
Over the course of treatment with NAC,
there were no adverse effects such as
dysrhythmias, bronchospasm, dizziness,
vomiting and rashes.
Gan 2013(66)
Malaysia
Case report
Post-dengue fever
shock syndrome
with fulminant
liver failure
(pediatric age 8
months)
1
IV NAC 10 mg/kg/hr.
Infant with severe dengue given
paracetamol; developed fulminant liver
failure with encephalopathy,
gastrointestinal hemorrhage, and severe
coagulopathy. Serum paracetamol was 0.1
mmol/l above the treatment nomogram at
20 hours after last dose of paracetamol.
Patient received supportive care
(transfusion, IV vitamin K) and NAC;
discharged at day 25 with lingering left
hemiparesis. No residual neurological
deficit at two months after initial illness.
Presumed
positive
effect
Key: ALF acute liver failure; ALI acute lung injury; ALP = alkaline phosphatase; ALT = alanine aminotransferase; ARDS acute respiratory distress syndrome; AST =
aspartate aminotransferase; AVH acute viral hepatitis; CABG coronary artery bypass grafting; COPD chronic obstructive pulmonary disease; CVVHD continuous veno-
venous hemodialysis; DF dengue fever; DIH drug induced hepatotoxicity; GGT gamma-glutamyl transferase; HCV = hepatitis C virus; ICU intensive care unit; IFN =
interferon; G-CSF granulocyte colony stimulating factor; ; INR international normalized ratio; MAP mean arterial pressure; MEGX = monoethylglycinexylidide; MET
metformin; NAC = N-acetylcysteine; NAFLD nonalcoholic fatty liver disease; PPC postoperative pulmonary complications; PT prothrombin time; RCT = randomized
controlled trial; SMT standard medical therapy; TB tuberculosis
31
14. Comprehensive reference list and in-text citations.
1. de Andrade KQ, Moura FA, dos Santos JM, de Araújo ORP, de Farias Santos JC, Goulart MOF.
Oxidative Stress and Inflammation in Hepatic Diseases: Therapeutic Possibilities of N-
Acetylcysteine. Int J Mol Sci. 2015 Dec 18;16(12):30269–308.
2. Sklar GE, Subramaniam M. Acetylcysteine treatment for non-acetaminophen-induced acute liver
failure. Ann Pharmacother. 2004 Mar;38(3):498–500.
3. Sales I, Dzierba AL, Smithburger PL, Rowe D, Kane-Gill SL. Use of acetylcysteine for non-
acetaminophen-induced acute liver failure. Ann Hepatol. 2013 Feb;12(1):6–10.
4. Hu J, Zhang Q, Ren X, Sun Z, Quan Q. Efficacy and safety of acetylcysteine in “non-
acetaminophen” acute liver failure: A meta-analysis of prospective clinical trials. Clin Res Hepatol
Gastroenterol. 2015 Oct;39(5):594–9.
5. Kawaji A, Sone T, Natsuki R, Isobe M, Takabatake E, Yamaura Y. In vitro toxicity test of poisonous
mushroom extracts with isolated rat hepatocytes. J Toxicol Sci. 1990 Aug;15(3):145–56.
6. Habashy WS, Milfort MC, Rekaya R, Aggrey SE. Cellular antioxidant enzyme activity and
biomarkers for oxidative stress are affected by heat stress. Int J Biometeorol. 2019
Dec;63(12):1569–84.
7. Dikici I, Mehmetoglu I, Dikici N, Bitirgen M, Kurban S. Investigation of oxidative stress and some
antioxidants in patients with acute and chronic viral hepatitis B and the effect of interferon-alpha
treatment. Clin Biochem. 2005 Dec;38(12):1141–4.
8. Guerri C, Grisolía S. Changes in glutathione in acute and chronic alcohol intoxication. Pharmacol
Biochem Behav. 1980;13 Suppl 1:53–61.
9. Choi DW, Kim SY, Kim SK, Kim YC. Factors involved in hepatic glutathione depletion induced by
acute ethanol administration. J Toxicol Environ Health A. 2000 Aug 11;60(7):459–69.
10. Calabrese V, Testa G, Ravagna A, Bates TE, Stella AM. HSP70 induction in the brain following
ethanol administration in the rat: regulation by glutathione redox state. Biochem Biophys Res
Commun. 2000 Mar 16;269(2):397–400.
11. Vogt BL, Richie JP. Glutathione Depletion and Recovery After Acute Ethanol Administration in the
Aging Mouse. Biochem Pharmacol. 2007 May 15;73(10):1613–21.
12. Strubelt O, Younes M, Pentz R. Enhancement by glutathione depletion of ethanol-induced acute
hepatotoxicity in vitro and in vivo. Toxicology. 1987 Aug;45(2):213–23.
13. Loguercio C, Federico A. Oxidative stress in viral and alcoholic hepatitis. Free Radic Biol Med.
2003 Jan 1;34(1):1–10.
32
14. Laitano O, Kalsi KK, Pook M, Oliveira AR, González-Alonso J. Separate and combined effects of
heat stress and exercise on circulatory markers of oxidative stress in euhydrated humans. Eur J
Appl Physiol. 2010 Nov;110(5):953–60.
15. Hillman AR, Vince RV, Taylor L, McNaughton L, Mitchell N, Siegler J. Exercise-induced dehydration
with and without environmental heat stress results in increased oxidative stress. Appl Physiol
Nutr Metab Physiol Appl Nutr Metab. 2011 Oct;36(5):698–706.
16. Rodrigues DF, Pires das Neves R, Carvalho ATP, Lourdes Bastos M, Costa VM, Carvalho F. In vitro
mechanistic studies on α-amanitin and its putative antidotes. Arch Toxicol. 2020;94(6):2061–78.
17. Fernando S, Wijewickrama A, Gomes L, Punchihewa CT, Madusanka SDP, Dissanayake H, et al.
Patterns and causes of liver involvement in acute dengue infection. BMC Infect Dis. 2016 Jul
8;16(1):319.
18. Sreekanth GP, Panaampon J, Suttitheptumrong A, Chuncharunee A, Bootkunha J,
Yenchitsomanus P, et al. Drug repurposing of N-acetyl cysteine as antiviral against dengue virus
infection. Antiviral Res. 2019 Jun;166:42–55.
19. Whillier S, Raftos JE, Chapman B, Kuchel PW. Role of N-acetylcysteine and cystine in glutathione
synthesis in human erythrocytes. Redox Rep. 2009 Jun 1;14(3):115–24.
20. Chandrasena L, Silva AD, Mel CD, Peiris H, Abesuriya V, Mel SD, et al. Glutathione Enzymes and
Liver Injury in Acute Dengue Viral Infection. J Biosci Med. 2019 Sep 29;7(10):61–71.
21. Bass S, Zook N. Intravenous acetylcysteine for indications other than acetaminophen overdose.
Am J Health Syst Pharm. 2013 Sep 1;70(17):1496–501.
22. Chertoff J. N-Acetylcysteine’s Role in Sepsis and Potential Benefit in Patients With
Microcirculatory Derangements. J Intensive Care Med. 2018 Feb;33(2):87–96.
23. Khan R, Koppe S. Modern Management of Acute Liver Failure. Gastroenterol Clin North Am. 2018
Jun;47(2):313–26.
24. Carrion AF, Martin P. Non–Intensive Care Unit Management of Acute Liver Failure. Clin Liver Dis.
2018 May;22(2):389–401.
25. Algren DA. Review of N-Acetylcysteine for the Treatment of Acetaminophen (paracetamol)
Toxicity in Pediatrics. Second Meeting of the Subcommittee of the Expert Committee on the
Selection and Use of Essential Medicines, 29 September to 3 October 2008. [Internet]. 2008.
Available from:
https://www.who.int/selection_medicines/committees/subcommittee/2/acetylcysteine_rev.pdf
26. Jones AL, Jarvie DR, Simpson D, Hayes PC, Prescott LF. Pharmacokinetics of N-acetylcysteine are
altered in patients with chronic liver disease. Aliment Pharmacol Ther. 1997 Aug;11(4):787–91.
27. ACETADOTE (acetylcysteine) Injection [Internet]. FDA. [cited 2020 Jan 17]. Available from:
https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021539s012lbl.pdf
33
28. Amin AF, Shaaban OM, Bediawy MA. N-acetyl cysteine for treatment of recurrent unexplained
pregnancy loss. Reprod Biomed Online. 2008 Nov;17(5):722–6.
29. Roes EM, Raijmakers MT, Boo TM de, Zusterzeel PL, Merkus HM, Peters WH, et al. Oral N-
acetylcysteine administration does not stabilise the process of established severe preeclampsia.
Eur J Obstet Gynecol Reprod Biol. 2006 Jul;127(1):61–7.
30. Jenkins DD, Wiest DB, Mulvihill DM, Hlavacek AM, Majstoravich SJ, Brown TR, et al. Fetal and
Neonatal Effects of N-Acetylcysteine When Used for Neuroprotection in Maternal
Chorioamnionitis. J Pediatr. 2016 Jan;168:67-76.e6.
31. Rumiris D, Purwosunu Y, Wibowo N, Farina A, Sekizawa A. Lower rate of preeclampsia after
antioxidant supplementation in pregnant women with low antioxidant status. Hypertens
Pregnancy. 2006;25(3):241–53.
32. Shahin AY, Hassanin IMA, Ismail AM, Kruessel JS, Hirchenhain J. Effect of oral N-acetyl cysteine on
recurrent preterm labor following treatment for bacterial vaginosis. Int J Gynaecol Obstet Off
Organ Int Fed Gynaecol Obstet. 2009 Jan;104(1):44–8.
33. Manka P, Verheyen J, Gerken G, Canbay A. Liver Failure due to Acute Viral Hepatitis (A-E). Visc
Med. 2016 Apr;32(2):80–5.
34. Dengue and severe dengue [Internet]. [cited 2020 Jan 13]. Available from:
https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue
35. Filho WL, Scheday S, Boenecke J, Gogoi A, Maharaj A, Korovou S. Climate Change, Health and
Mosquito-Borne Diseases: Trends and Implications to the Pacific Region. Int J Environ Res Public
Health. 2019 14;16(24).
36. Pathak VK, Mohan M. A notorious vector-borne disease: Dengue fever, its evolution as public
health threat. J Fam Med Prim Care. 2019 Oct 31;8(10):3125–9.
37. Wichmann O, Hongsiriwon S, Bowonwatanuwong C, Chotivanich K, Sukthana Y, Pukrittayakamee
S. Risk factors and clinical features associated with severe dengue infection in adults and children
during the 2001 epidemic in Chonburi, Thailand. Trop Med Int Health TM IH. 2004 Sep;9(9):1022–
9.
38. Wu X, Brady JE, Rosenberg H, Li G. Emergency Department Visits for Heat Stroke in the United
States, 2009 and 2010. Inj Epidemiol [Internet]. 2014 Apr 24 [cited 2020 Nov 27];1(1). Available
from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5005673/
39. WHO | Heatwaves and health: guidance on warning-system development [Internet]. WHO.
World Health Organization; [cited 2020 Nov 27]. Available from:
https://www.who.int/globalchange/publications/heatwaves-health-guidance/en/
40. Acute Liver Failure Caused by Amanita phalloides Poisoning [Internet]. [cited 2020 Nov 27].
Available from: https://www.hindawi.com/journals/ijh/2012/487480/
41. Karlson-Stiber C, Persson H. Cytotoxic fungi—an overview. Toxicon. 2003 Sep 1;42(4):339–49.
34
42. Mathurin P, Mendenhall CL, Carithers RL, Ramond M-J, Maddrey WC, Garstide P, et al.
Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis (AH):
individual data analysis of the last three randomized placebo controlled double blind trials of
corticosteroids in severe AH. J Hepatol. 2002 Apr;36(4):480–7.
43. Naveau S, Giraud V, Borotto E, Aubert A, Capron F, Chaput JC. Excess weight risk factor for
alcoholic liver disease. Hepatol Baltim Md. 1997 Jan;25(1):108–11.
44. Global status report on alcohol and health 2018 [Internet]. [cited 2020 Nov 27]. Available from:
https://www.who.int/publications-detail-redirect/9789241565639
45. Nabi T, Nabi S, Rafiq N, Shah A. Role of N-acetylcysteine treatment in non-acetaminophen-
induced acute liver failure: A prospective study. Saudi J Gastroenterol Off J Saudi Gastroenterol
Assoc. 2017 Jun;23(3):169–75.
46. Reuben A, Tillman H, Fontana RJ, Davern T, McGuire B, Stravitz RT, et al. Outcomes in Adults With
Acute Liver Failure Between 1998 and 2013: An Observational Cohort Study. Ann Intern Med.
2016 Jun 7;164(11):724.
47. Monzon B, Hegarty K, Rech MA. A knack for “NAC”: Treatment for heat stroke induced acute liver
injury. Am J Emerg Med. 2020;38(4):853.e1-853.e3.
48. Aquilina A, Pirotta T, Aquilina A. Acute liver failure and hepatic encephalopathy in exertional heat
stroke. BMJ Case Rep. 2018 Jul 30;2018.
49. Will JS, Snyder CJ, Westerfield KL. N-Acetylcysteine (NAC) for the Prevention of Liver Failure in
Heat Injury-Mediated Ischemic Hepatitis. Mil Med. 2019 01;184(9–10):565–7.
50. Singh S, Murad MH, Chandar AK, Bongiorno CM, Singal AK, Atkinson SR, et al. Comparative
Effectiveness of Pharmacological Interventions for Severe Alcoholic Hepatitis: A Systematic
Review and Network Meta-analysis. Gastroenterology. 2015 Oct;149(4):958-970.e12.
51. Liu J, Chen Y, Gao Y, Walline JH, Lu X, Yu S, et al. N-acetylcysteine as a treatment for amatoxin
poisoning: a systematic review. Clin Toxicol Phila Pa. 2020 Nov;58(11):1015–22.
52. Saleem AF, Abbas Q, Haque AU. Use of N-acetylcysteine in children with fulminant hepatic failure
caused by acute viral hepatitis. J Coll Physicians Surg--Pak JCPSP. 2015 May;25(5):354–8.
53. Sotelo N, de los Angeles Durazo M, Gonzalez A, Dhanakotti N. Early treatment with N-
acetylcysteine in children with acute liver failure secondary to hepatitis A. Ann Hepatol. 2009
Dec;8(4):353–8.
54. Habaragamuwa BWP, Dissanayaka P. N-acetylcystein in dengue associated severe hepatitis.
Indian J Crit Care Med. 2014 Mar;18(3):181–2.
55. Paramasivam R, Balakrishnan T, Choo KS, Kl Z, Dass EP, Mokhtar H, et al. Viral Hepatitis ( plus
Antiviral Therapy) Outcome of hepatitis in dengue after administration of N-acetylcysteine in
Klang Hospital. J Gastroenterol Hepatol. 2013 Oct;28:418–9.
35
56. Dalugama C, Gawarammana IB. Lessons learnt from managing a case of dengue hemorrhagic
fever complicated with acute liver failure and acute kidney injury: a case report. J Med Case
Reports. 2018 Aug 8;12(1):215.
57. Tan S-S, Bujang MA. The clinical features and outcomes of acute liver failure associated with
dengue infection in adults: a case series. Braz J Infect Dis. 2013 Apr;17(2):164–9.
58. Kumarasena RS, Mananjala Senanayake S, Sivaraman K, de Silva AP, Dassanayake AS, Premaratna
R, et al. Intravenous N-acetylcysteine in dengue-associated acute liver failure. Hepatol Int. 2010
Jun;4(2):533–4.
59. Senanayake MP, Jayamanne MDCJP, Kankananarachchi I. N-acetylcysteine in children with acute
liver failure complicating dengue viral infection. Ceylon Med J. 2013 Jun;58(2):80–2.
60. Kularatne SAM, Ralapanawa U, Dalugama C, Jayasinghe J, Rupasinghe S, Kumarihamy P. Series of
10 dengue fever cases with unusual presentations and complications in Sri Lanka: a single centre
experience in 2016. BMC Infect Dis. 2018 Dec 18;18(1):674.
61. Tan JMC, Tan NWH, Thoon KC, Chong CY, Ong C. Dengue Fever Associated Liver Failure. Pediatr
Infect Dis Open Access [Internet]. 2016 Dec 1 [cited 2020 Jan 13];1(4). Available from:
http://pediatric-infectious-disease.imedpub.com/abstract/dengue-fever-associated-liver-failure-
17598.html
62. Lewis J, Mitra A, Chang MF. Mo1425 – Acute Liver Failure in a Patient with Dengue Shock
Syndrome. Gastroenterology. 2019 May 1;156(6):S.
63. Dalugama C, Gawarammana IB. Dengue hemorrhagic fever complicated with acute liver failure: a
case report. J Med Case Reports. 2017 Dec;11(1):341.
64. Abeysekera RA, Illangasekera U, Jayalath T, Sandeepana AGW, Kularatne S a. M. Successful use of
intravenous N-acetylcysteine in dengue haemorrhagic fever with acute liver failure. Ceylon Med
J. 2012 Dec;57(4):166–7.
65. Lim G, Lee JH. N-acetylcysteine in Children with Dengue-associated Liver Failure: A Case Report. J
Trop Pediatr. 2011 Dec 23;58(5):409–13.
66. Gan CS, Chong SY, Lum LCS, Lee WS. Regular paracetamol in severe dengue: a lethal
combination? Singapore Med J. 2013 Feb;54(2):e35-37.
67. Manoj EM, Ranasinghe G, Ragunathan MK. Successful use of N-acetyl cysteine and activated
recombinant factor VII in fulminant hepatic failure and massive bleeding secondary to dengue
hemorrhagic fever. J Emerg Trauma Shock. 2014 Oct;7(4):313–5.
68. Denny JC, Ritchie MD, Basford MA, Pulley JM, Bastarache L, Brown-Gentry K, et al. PheWAS:
demonstrating the feasibility of a phenome-wide scan to discover gene-disease associations.
Bioinforma Oxf Engl. 2010 May 1;26(9):1205–10.
36
69. Pulley JM, Shirey-Rice JK, Lavieri RR, Jerome RN, Zaleski NM, Aronoff DM, et al. Accelerating
Precision Drug Development and Drug Repurposing by Leveraging Human Genetics. ASSAY Drug
Dev Technol. 2017 Apr;15(3):113–9.
70. WHO model list of essential medicines [Internet]. [cited 2020 Nov 20]. Available from:
https://www.who.int/publications-detail-redirect/WHOMVPEMPIAU2019.06
71. Lee WM, Hynan LS, Rossaro L, Fontana RJ, Stravitz RT, Larson AM, et al. Intravenous N-
acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver
failure. Gastroenterology. 2009 Sep;137(3):856-864.e1.
72. Singh S, Hynan LS, Lee WM, Acute Liver Failure Study Group. Improvements in hepatic serological
biomarkers are associated with clinical benefit of intravenous N-acetylcysteine in early stage non-
acetaminophen acute liver failure. Dig Dis Sci. 2013 May;58(5):1397–402.
73. Stravitz RT, Sanyal AJ, Reisch J, Bajaj JS, Mirshahi F, Cheng J, et al. Effects of N -acetylcysteine on
cytokines in non-acetaminophen acute liver failure: potential mechanism of improvement in
transplant-free survival. Liver Int. 2013 Oct;33(9):1324–31.
74. Darweesh SK, Ibrahim MF, El-Tahawy MA. Effect of N-Acetylcysteine on Mortality and Liver
Transplantation Rate in Non-Acetaminophen-Induced Acute Liver Failure: A Multicenter Study.
Clin Drug Investig. 2017 May;37(5):473–82.
75. Kortsalioudaki C, Taylor RM, Cheeseman P, Bansal S, Mieli-Vergani G, Dhawan A. Safety and
efficacy of N-acetylcysteine in children with non-acetaminophen-induced acute liver failure. Liver
Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2008 Jan;14(1):25–30.
76. Mumtaz K, Azam Z, Hamid S, Abid S, Memon S, Ali Shah H, et al. Role of N-acetylcysteine in adults
with non-acetaminophen-induced acute liver failure in a center without the facility of liver
transplantation. Hepatol Int. 2009 Dec;3(4):563–70.
77. Ben-Ari Z, Vaknin H, Tur-Kaspa R. N-acetylcysteine in acute hepatic failure (non-paracetamol-
induced). Hepatogastroenterology. 2000 Jun;47(33):786–9.
78. Harrison PM, Wendon JA, Gimson AE, Alexander GJ, Williams R. Improvement by acetylcysteine
of hemodynamics and oxygen transport in fulminant hepatic failure. N Engl J Med. 1991 Jun
27;324(26):1852–7.
79. Karvellas CJ, Tillman H, Leung AA, Lee WM, Schilsky ML, Hameed B, et al. Acute liver injury and
acute liver failure from mushroom poisoning in North America. Liver Int Off J Int Assoc Study
Liver. 2016;36(7):1043–50.
80. Vanooteghem S, Arts J, Decock S, Pieraerts P, Meersseman W, Verslype C, et al. Four patients
with Amanita Phalloides poisoning. Acta Gastro-Enterol Belg. 2014 Sep;77(3):353–6.
81. Montaninia S, Sinardia D, Praticò C, Sinardia A, Trimarchi G. Use of Acetylcysteine as the Life-
saving Antidote in Amanita phalloides (Death Cap) Poisoning. Arzneimittelforschung. 2011 Dec
28;49(12):1044–7.
37
82. A Multi-center Comparison of the Safety of Oral versus Intravenous Acetylcysteine for Treatment
of Acetaminophen Overdose. Clin Toxicol Phila Pa. 2010 Jun;48(5):424–30.
83. Sandilands EA, Bateman DN. Adverse reactions associated with acetylcysteine. Clin Toxicol. 2009
Feb 1;47(2):81–8.
84. Ershad M, Vearrier D. N Acetylcysteine. In: StatPearls [Internet]. Treasure Island (FL): StatPearls
Publishing; 2019 [cited 2020 Jan 7]. Available from:
http://www.ncbi.nlm.nih.gov/books/NBK537183/
85. Yarema M, Chopra P, Sivilotti MLA, Johnson D, Nettel-Aguirre A, Bailey B, et al. Anaphylactoid
Reactions to Intravenous N-Acetylcysteine during Treatment for Acetaminophen Poisoning. J
Med Toxicol. 2018 Jun;14(2):120–7.
86. Pakravan N, Waring WS, Sharma S, Ludlam C, Megson I, Bateman DN. Risk factors and
mechanisms of anaphylactoid reactions to acetylcysteine in acetaminophen overdose. Clin
Toxicol. 2008 Jan 1;46(8):697–702.
87. Schmidt LE, Dalhoff K. Risk factors in the development of adverse reactions to N-acetylcysteine in
patients with paracetamol poisoning. Br J Clin Pharmacol. 2001 Jan;51(1):87–91.
88. Waring WS, Stephen AF, Robinson OD, Dow MA, Pettie JM. Lower incidence of anaphylactoid
reactions to N-acetylcysteine in patients with high acetaminophen concentrations after overdose.
Clin Toxicol Phila Pa. 2008 Jul;46(6):496–500.
89. British National Formulary (BNF) 69. :1197.
90. Appelboam AV, Dargan PI, Knighton J. Fatal anaphylactoid reaction to N-acetylcysteine: caution in
patients with asthma. Emerg Med J. 2002 Nov 1;19(6):594–5.
91. CETYLEV (acetylcysteine) effervescent tablets for oral solution (FDA package insert). [Internet].
FDA. [cited 2020 Jan 17]. Available from:
https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/207916s000lbl.pdf
92. Mullins ME, Schmidt RU, Jang TB. What is the rate of adverse events with intravenous versus oral
N-acetylcysteine in pediatric patients? Ann Emerg Med. 2004 Nov 1;44(5):547–8.
93. Deepmala, Slattery J, Kumar N, Delhey L, Berk M, Dean O, et al. Clinical trials of N-acetylcysteine
in psychiatry and neurology: A systematic review. Neurosci Biobehav Rev. 2015 Aug 1;55:294–
321.
94. Pereira JEG, Dib RE, Braz LG, Escudero J, Hayes J, Johnston BC. N-acetylcysteine use among
patients undergoing cardiac surgery: A systematic review and meta-analysis of randomized trials.
PLOS ONE. 2019 May 9;14(5):e0213862.
95. Fen F, Zhang J, Wang Z, Wu Q, Zhou X. Efficacy and safety of N‑acetylcysteine therapy for
idiopathic pulmonary fibrosis: An updated systematic review and meta‑analysis. Exp Ther Med.
2019 Jul 1;18(1):802–16.
38
96. Pannu N, Manns B, Lee H, Tonelli M. Systematic review of the impact of N-acetylcysteine on
contrast nephropathy. Kidney Int. 2004 Apr 1;65(4):1366–74.
97. Pauley KA, Sandritter TL, Lowry JA, Algren DA. Evaluation of an Alternative Intravenous N-
Acetylcysteine Regimen in Pediatric Patients. J Pediatr Pharmacol Ther JPPT. 2015;20(3):178–85.
98. PubChem. Acetylcysteine [Internet]. [cited 2020 Jan 20]. Available from:
https://pubchem.ncbi.nlm.nih.gov/compound/12035
99. Šalamon Š, Kramar B, Marolt TP, Poljšak B, Milisav I. Medical and Dietary Uses of N-
Acetylcysteine. Antioxidants [Internet]. 2019 Apr 28 [cited 2020 Jan 8];8(5). Available from:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6562654/
100. Keays R, Harrison PM, Wendon JA, Forbes A, Gove C, Alexander GJ, et al. Intravenous
acetylcysteine in paracetamol induced fulminant hepatic failure: a prospective controlled trial.
BMJ. 1991 Oct 26;303(6809):1026–9.
101. Kerr F, Dawson A, Whyte IM, Buckley N, Murray L, Graudins A, et al. The Australasian Clinical
Toxicology Investigators Collaboration Randomized Trial of Different Loading Infusion Rates of N-
Acetylcysteine. Ann Emerg Med. 2005 Apr 1;45(4):402–8.
102. Bonfiglio MF, Traeger SM, Hulisz DT, Martin BR. Anaphylactoid Reaction to Intravenous
Acetylcysteine Associated with Electrocardiographic Abnormalities. Ann Pharmacother. 1992 Jan
1;26(1):22–5.
103. Bailey B, Blais R, Letarte A. Status epilepticus after a massive intravenous N-acetylcysteine
overdose leading to intracranial hypertension and death. Ann Emerg Med. 2004 Oct 1;44(4):401–
6.
104. Mohammed S, Jamal AZ, Robison LR. Serum Sickness-Like Illness Associated with N-
Acetyicysteine Therapy. Ann Pharmacother. 1994 Feb 1;28(2):285–285.
105. Acetylcysteine 200mg/ml Injection - Summary of Product Characteristics (SmPC) - (emc)
[Internet]. [cited 2020 Jan 24]. Available from:
https://www.medicines.org.uk/emc/product/3447/smpc
106. ACETYLCYSTEINE SOLUTION, USP [Internet]. [cited 2020 Jan 24]. Available from:
https://docs.boehringer-
ingelheim.com/Prescribing%20Information/PIs/Roxane/Acetylcysteine/Acetylcysteine%20Solutio
n%20USP.pdf
107. Parvolex (Acetylcysteine) 200 mg/ml Concentrate for Solution for Infusion - Summary of Product
Characteristics (SmPC) - (emc) [Internet]. [cited 2020 Jan 24]. Available from:
https://www.medicines.org.uk/emc/product/1000/smpc
108. Cook M, Zavala E. The finances of a liver transplant program. Curr Opin Organ Transplant.
2019;24(2):156–60.
39
109. Hilst CS van der, IJtsma AJC, Slooff MJH, TenVergert EM. Cost of Liver Transplantation: A
Systematic Review and Meta-Analysis Comparing the United States With Other OECD Countries.
Med Care Res Rev [Internet]. 2008 Sep 16 [cited 2020 Nov 25]; Available from:
https://journals.sagepub.com/doi/10.1177/1077558708324299
110. Squires RH, Dhawan A, Alonso E, Narkewicz MR, Shneider BL, Rodriguez-Baez N, et al.
Intravenous N-acetylcysteine in pediatric patients with nonacetaminophen acute liver failure: a
placebo-controlled clinical trial. Hepatol Baltim Md. 2013 Apr;57(4):1542–9.
111. Grant PR, Black A, Garcia N, Prieto J, Garson JA. Combination therapy with interferon-alpha plus
N-acetyl cysteine for chronic hepatitis C: a placebo controlled double-blind multicentre study. J
Med Virol. 2000 Aug;61(4):439–42.
112. Neri S, Ierna D, Antoci S, Campanile E, D’Amico RA, Noto R. Association of alpha-interferon and
acetyl cysteine in patients with chronic C hepatitis. Panminerva Med. 2000 Sep;42(3):187–92.
113. Gunduz H, Karabay O, Tamer A, Ozaras R, Mert A, Tabak OF. N-acetyl cysteine therapy in acute
viral hepatitis. World J Gastroenterol. 2003 Dec;9(12):2698–700.
114. Look MP, Gerard A, Rao GS, Sudhop T, Fischer HP, Sauerbruch T, et al. Interferon/antioxidant
combination therapy for chronic hepatitis C--a controlled pilot trial. Antiviral Res. 1999
Sep;43(2):113–22.
115. Furtado I, Valadares D, Nery FG. Acute hepatitis B virus infection and severe non-immune
haemolytic anaemia: a rare relationship. BMJ Case Rep. 2017 Oct 24;2017.
116. Baniasadi S, Eftekhari P, Tabarsi P, Fahimi F, Raoufy MR, Masjedi MR, et al. Protective effect of N-
acetylcysteine on antituberculosis drug-induced hepatotoxicity. Eur J Gastroenterol Hepatol.
2010 Oct;22(10):1235–8.
117. Cheng S-L. Protective effect of N-acetylcysteine on antituberculosis drug-induced hepatotoxicity.
Eur Respir J [Internet]. 2016 Sep 1 [cited 2020 Jan 10];48(suppl 60). Available from:
https://erj.ersjournals.com/content/48/suppl_60/PA2716
118. Oliveira CP, Cotrim HP, Stefano JT, Siqueira ACG, Salgado ALA, Parise ER. N-ACETYLCYSTEINE
AND/OR URSODEOXYCHOLIC ACID ASSOCIATED WITH METFORMIN IN NON-ALCOHOLIC
STEATOHEPATITIS: AN OPEN-LABEL MULTICENTER RANDOMIZED CONTROLLED TRIAL. Arq
Gastroenterol. 2019 Aug 13;56(2):184–90.
119. Singh V, Keisham A, Bhalla A, Sharma N, Agarwal R, Sharma R, et al. Efficacy of Granulocyte
Colony-Stimulating Factor and N-Acetylcysteine Therapies in Patients With Severe
Alcoholic Hepatitis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2018
Oct;16(10):1650-1656.e2.
120. Kakaei F, Fasihi M, Hashemzadeh S, Zarrintan S, Beheshtirouy S, Asvadi-Kermani T, et al. Effect of
N-acetylcysteine on liver and kidney function tests after surgical bypass in obstructive jaundice: A
randomized controlled trial. Asian J Surg. 2019 Jul 4;
40
121. Li X, Wei X, Chen C, Zhang Z, Liu D, Hei Z, et al. N-Acetylcysteine inhalation improves pulmonary
function in patients received liver transplantation. Biosci Rep. 2018 31;38(5).
122. Aliakbarian M, Nikeghbalian S, Ghaffaripour S, Bahreini A, Shafiee M, Rashidi M, et al. Effects of
N-Acetylcysteine Addition to University of Wisconsin Solution on the Rate of Ischemia-
Reperfusion Injury in Adult Orthotopic Liver Transplant. Exp Clin Transplant Off J Middle East Soc
Organ Transplant. 2017 Aug;15(4):432–6.
123. Grendar J, Ouellet JF, McKay A, Sutherland FR, Bathe OF, Ball CG, et al. Effect of N-acetylcysteine
on liver recovery after resection: A randomized clinical trial. J Surg Oncol. 2016 Sep;114(4):446–
50.
124. Donadon M, Molinari AF, Corazzi F, Rocchi L, Zito P, Cimino M, et al. Pharmacological Modulation
of Ischemic-Reperfusion Injury during Pringle Maneuver in Hepatic Surgery. A Prospective
Randomized Pilot Study. World J Surg. 2016 Sep;40(9):2202–12.
125. D’Amico F, Vitale A, Piovan D, Bertacco A, Ramirez Morales R, Chiara Frigo A, et al. Use of N-
acetylcysteine during liver procurement: a prospective randomized controlled study. Liver
Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2013 Feb;19(2):135–44.
126. Robinson SM, Saif R, Sen G, French JJ, Jaques BC, Charnley RM, et al. N-acetylcysteine
administration does not improve patient outcome after liver resection. HPB. 2013 Jun;15(6):457–
62.
127. Regueira FM, Cienfuegos JA, Pardo F, Hernández JL, Díez-Caballero A, Sierra A, et al.
Improvement in early function of the hepatic graft after treatment of the donor with N-
acetylcysteine: clinical study. Transplant Proc. 1997 Dec;29(8):3350–2.
128. Hamamsy ME, Bondok R, Shaheen S, Eladly GH. Safety and efficacy of adding intravenous N-
acetylcysteine to parenteral L-alanyl-L-glutamine in hospitalized patients undergoing surgery of
the colon: a randomized controlled trial. Ann Saudi Med. 2019 Aug;39(4):251–7.
129. Onk D, Özçelik F, Onk OA, Günay M, Akarsu Ayazoğlu T, Ünver E. Assessment of Renal and
Hepatic Tissue-Protective Effects of N-Acetylcysteine via Ammonia Metabolism: A Prospective
Randomized Study. Med Sci Monit Int Med J Exp Clin Res. 2018 Mar 15;24:1540–6.
130. Belgaumkar AP, Carswell KA, Hughes RD, Quaglia A, Dhawan A, Mitry RR, et al. The Effect of
Intraoperative N-Acetylcysteine on Hepatocellular Injury During Laparoscopic Bariatric Surgery. A
Randomised Controlled Trial. Obes Surg. 2016 Jun;26(6):1254–65.
131. Rank N, Michel C, Haertel C, Lenhart A, Welte M, Meier-Hellmann A, et al. N-acetylcysteine
increases liver blood flow and improves liver function in septic shock patients: results of a
prospective, randomized, double-blind study. Crit Care Med. 2000 Dec;28(12):3799–807.
132. Schmidt LE, Knudsen TT, Dalhoff K, Bendtsen F. Effect of acetylcysteine on prothrombin index in
paracetamol poisoning without hepatocellular injury. Lancet Lond Engl. 2002 Oct
12;360(9340):1151–2.
41
133. Gray KM, Watson NL, Carpenter MJ, LaRowe SD. N-Acetylcysteine (NAC) in Young Marijuana
Users: An Open-Label Pilot Study. Am J Addict. 2010;19(2):187–9.
134. The effect of oral N-acetylecysteine in chronic bronchitis: a quantitative systematic review.
Available from: https://erj.ersjournals.com/content/erj/16/2/253.full.pdf
135. Naveed S, Amray A, Waqas A, Chaudhary AM, Azeem MW. Use of N-Acetylcysteine in Psychiatric
Conditions among Children and Adolescents: A Scoping Review. Cureus [Internet]. [cited 2020 Jan
20];9(11). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5788395/
Appendix 1: Additional contributors
The following individuals contributed content, editing, and/or scientific review to the current
application:
Robert Wallis, M.D., FIDSA, Chief Scientific Officer, The AURUM Institute, Johannesburg, South Africa
Gordon Bernard, M.D., Executive Vice President for Research, Senior Associate Dean for Clinical
Sciences, Vanderbilt University Medical Center, Nashville, TN, USA,
Jana Shirey-Rice, Ph.D., Team Lead, Drug Repurposing, Vanderbilt Institute for Clinical and
Translational Research, Vanderbilt University Medical Center, Nashville, TN, USA
Nicole Zaleski, MA, MPH, Project Manager, Vanderbilt Institute for Clinical and Translational
Research, Vanderbilt University Medical Center, Nashville, TN, USA
Laura Zahn, M.S., Research Services Consultant, Vanderbilt Institute for Clinical and Translational
Research, Vanderbilt University Medical Center, Nashville, TN, USA
Nan Kennedy, Program Manager, Vanderbilt Institute for Clinical and Translational Research,
Vanderbilt University Medical Center, Nashville, TN, USA

42
Appendix 2: Evidence describing hepatic effects of N- acetylcysteine in other conditions (excluding
acetaminophen toxicity)
First author, year
country
Design
Condition
Sample
size
NAC dose, frequency,
duration, route of
administration;
comparator
Findings
Outcome
Other infections
Grant, 2000(111)
UK
Double blind,
multicenter
RCT
Chronic
hepatitis C
147
Oral NAC 600 mg 3
times/day for 6 months
(1800 mg/day)
73 received 3MU IFN-
alpha 3 times/week by
subcutaneous injection +
NAC 3 times/day and 74
received IFN 3
times/week + placebo 3
times/day
No significant difference in sustained
virological response; Changes in serum ALT
levels correlated with virological outcome in
97% (n = 139) of cases; in 5 patients, serum
ALT remained normal despite virologic
relapse during 6 month post-treatment
window (paper does not report treatment
group data for these individuals).
no effect on
virologic response
Neri, 2000(112)
Italy
RCT
Chronic
hepatitis C
77
Participants were treated
with IFN (intramuscular
6,000,000 MU 3 times
each week for 6 months)
+ NAC (2400 mg/day in
two doses when fasting)
(n=38) or with IFN alone
(n=39)
No significant difference in viremia values
between the two groups. 7 patients from the
IFN only group and 6 patients from the
IFN+NAC group had no evidence of relapse
within 10 months after finishing therapy and
were excluded from further analysis.
Patients treated with IFN alone relapsed
earlier than patients treated with NAC + IFN
(22 vs. 31 weeks, p<0.05). Three cases of
breakthrough occurred in the IFN only group
and no cases in the IFN+NAC group.
Oxidase-reductase balance was significantly
higher during treatment in the IFN group as
compared with the IFN+NAC group (p<0.05),
which authors note suggests the presence of
oxidative stress in patients treated with IFN
alone. The difference waned over time after
cessation of treatment.
No adverse events requiring cessation of
either treatment.
Positive effect on
HCV relapse and
measures of
oxidative stress
no difference in
efficacy (ALT, liver
biopsy or
ultrasound)

43
Gunduz, 2003(113)
Turkey
Placebo
controlled RCT
Acute viral
hepatitis A or B
(hospitalized
patients)
41
Oral NAC 200 mg 3x daily
(600 mg/day) until
discharge
21 received placebo
tablets and 20 received
NAC
NAC administration did not significantly affect
the time necessary for ALT and total bilirubin
values to normalize (100 U/L and 2mg/dl,
respectively) or duration of hospitalization
“NAC was not harmful for AVH patients”
(given that hospital duration and time to
normalization of ALT and total bilirubin did
not get longer with use of NAC; no adverse
effects noted)
no effect on time
to normalization of
ALT or total
bilirubin; or
duration of
hospitalization
Look, 1999(114)
Germany
Prospective,
randomized
pilot trial
Chronic
hepatitis C
24
NAC effervescent tablets,
1800 mg/day for 24
weeks
8 received IFN alone, 8
received IFN + NAC +
sodium selenite, and 8
received IFN + NAC +
sodium selenite + vitamin
E544
NAC administration did not significantly affect
the response rate (normalization of ALT and
negative HCV RNA). No significant
improvement in liver histology or recurrence
rates.
no effect on
normalization of
ALT or negative
HCV RNA
Furtado 2017(115)
Portugal
Case report
Acute HBV and
coagulopathy
with non-
immune
hemolytic
anemia (age 41
years)
1
NAC for 5 days (protocol
not detailed), along with
entecavir, folic acid, and
vitamin K
Bilirubin and INR normalized progressively
during hospital stay.
Presumed positive
effect
Prevention and treatment of non-acetaminophen drug-induced acute liver injury and failure
Baniasadi,
2010(116)
Iran
Open-label,
RCT
Newly
diagnosed,
treatment
naïve,
pulmonary
tuberculosis
patients
beginning anti-
TB drug
regimen
60
600 mg NAC taken orally
twice a day
32 received standard anti-
TB drug therapy and 28
received standard drug
therapy + NAC
Hepatotoxicity occurred in 12 control patients
(37.5%) and none of the patients treated with
NAC.
After 1 and 2 weeks of treatment, mean ± SD
values of AST and ALT were significantly
higher in controls (week 1: 99.44±150.11,
65.78±88.64 and week 2: 57.22±75.81,
58.09±86.18) than those treated with NAC
(week 1: 27.68±13.79, 20.96±11.95, and week
2: 27.32±13.11, 21.53±9.56).
Positive effect
Cheng 2016(117)
Taiwan
Case control
Patients
receiving at
least 5 months
of an anti-TB
drug course
279
NAC 1200 mg/day plus
anti-TB regimen (n=82)
Anti-TB regimen only
(n=297)
11 patients (13.4%) were diagnosed with
drug-induced hepatotoxicity (DIH) in the NAC
group and 72 patients (24.2%) developed DIH
in the comparison group (p<0.05).
Positive effect

44
The mean duration of treatment before the
onset of hepatotoxicity was 28.6±14.9 days in
NAC group, as compared with 17.4±11.3 days
in the comparison group (p<0.01).
Liver function normalized 9.4 ±6.7 days after
stopping the anti-TB drugs in NAC group
compared with 19.2±11.5 days in the
comparison group (p<0.01).
Non-viral hepatitis
Oliveira, 2019(118)
Brazil
Open-label,
multicenter
RCT
Non-alcoholic
steatohepatitis
53
1200 mg/day of NAC
taken orally for 48 weeks
in combination with
metformin and/or
ursodeoxycholic acid
26 received metformin +
NAC + UDCA, 14 received
metformin + NAC, 13
received metformin +
UDCA
Significant improvements in the steatosis
degree (P=0.014), ballooning (0.027) and,
consequently, in the NAFLD Activity Score
(NAS) (P=0.005), and in the ALT levels at the
end of the treatment only in the metformin +
NAC group
No significant evidence of modification in the
liver fibrosis in any group (via baseline and
post-treatment liver biopsies)
Positive effect
Singh, 2018(119)
India
Open-label,
randomized
controlled pilot
study
Severe
alcoholic
hepatitis
57
Intravenous NAC for 5
days (day 1: NAC at 150,
50, and 100 mg/kg in 250,
500, and 1000 mL of 5%
glucose solution over 30
minutes, 4 hours, and 16
hours, respectively; days
2–5: 100 mg/kg/day in
1000 mL of 5% glucose
solution) in addition to
standard medical therapy
and G-CSF
20 received standard
medical therapy (SMT),
18 received SMT +
granulocyte colony-
stimulating factor (G-
CSF), and 19 received
SMT+ G-CSF + NAC
Both G-CSF and SMT+G-CSF + NAC improved
90-day survival compared to standard
medical therapy, but no significant difference
between this combination and G-CSF alone
*There was a significant increase in ALP on
day 6 compared with day 0 in the SMT+G-
CSF+NAC group and the G-CSF group; no
significant increase in ALP in the SMT alone
group.
There was significant improvement in the
clinical severity scores on day 6 compared
with day 0 in G-CSF group but not in the
SMT+G-CSF+NAC and the SMT group.
No effect
potential liver-
related safety issue
Hepatic surgical procedure (resection, transplant, biliary bypass)

45
Kakaei 2020(120)
Iran
RCT
Biliary bypass
for obstructive
jaundice
30
IV NAC 200 mg/kg/hour
first 8 hours after surgery,
then 100 mg/kg/hour for
40 hours
Control participants
received no infusion
The NAC group, as compared with control
patients, had significantly greater decline of
ALT (p=0.02), AST (p=0.01), ALP (p=0.01),
GGT (p=0.04), total bilirubin (p=0.02), direct
bilirubin (p=0.01). No significant differences
in decline of INR or creatinine or of hospital
stay duration, ICU admission, or ICU length of
stay were observed.
No adverse events due to NAC were observed
Positive effect
Li 2018(121)
China
RCT
Liver
transplantation
60 (42
included in
analysis)
Atomization inhalation of
3 mL NAC (10%) for 30
minutes before surgery
and 3 hours after
reperfusion
Control patients received
atomization inhalation of
3 ml sterile water for the
same periods of time.
Postoperative pulmonary complication as per
broad clinical definitions (atelectasis,
pneumothorax, pleural effusion, ALI, ARDS,
pneumonia) were similar between the two
groups. As measured by the Melbourne Group
Scale Version 2 score, there was a significantly
higher incidence of PPCs in the control group
as compared with the NAC group (73% vs.
40%, p=0.032).
30 participants developed at least one
respiratory infection within 1 month
postoperatively. Among these patients,
isolated bacterial infection was observed in 2
(11%) of 18 patients in the control group and
8 (67%) of 12 patients in the NAC group
(p=0.019), while investigators note that more
refractory infectious etiologies including
fungal, combined, or unknown infection
comprised the major pneumonia causes in the
control group but not the NAC group.
18 of the 22 control patients developed and
combined more than 3 kids of coexisting
pulmonary complications within 1 month
postoperatively, an incidence higher than that
of the NAC group (82% vs. 50%, p=0.028).
NAC group had significantly shorter ICU
(p=0.026) and hospital stay (p=0.029) and
hospitalization costs (p=0.020). 12 month
mortality related to pulmonary disease was
significantly lower in the NAC group
(p=0.012).
Positive effect
46
No side effects related to NAC inhalation were
observed.
Aliakbarian,
2017(122)
Iran
Double-blind
RCT
Orthotopic
liver transplant
115
After donor organ
retrieval, 2000 mg NAC
was added to 2 liters of
UW solution and perfused
through the mesenteric
vein (49 patients)
In 66 patients, standard
UW solution was used
No significant difference between the groups
regarding time to hepatic artery reperfusion,
hospital stay, vascular complications, inotrope
requirement before and after portal
declamping, and blood gas analysis. (no liver
specific measures)
*Hypotension after portal reperfusion was
significantly more common in experimental
group compared with control group (P =
.005).
Retransplant and in-hospital mortality were
comparable between the groups.
no effect
Grendar, 2016(123)
Canada
Open-label,
RCT (No
blinding was
performed)
Post-hepatic
resection
206
NAC infusion: 150 mg/kg
of NAC in 200 ml of 5%
glucose over 45 min upon
arrival in the post
anesthesia recovery room,
then 50 mg/kg of NAC in
500 ml of 5% glucose over
4 hr., followed by
100 mg/kg in 1000 ml of
5% glucose over the next
16 hr.; followed by
100 mg/kg in 1000 ml of
5% glucose per day over
the next 3 days, which
was administered in six
infusions of 50 mg/kg in
500 ml of 5% glucose,
given over 12 hr. each +
standard care (98
patients)
108 patients received the
usual postoperative
management without NAC
No significant differences were noted in
overall complications (32.7% and
45.7%, P = 0.06) or hepatic failure (3.6% and
5.4%, P = 0.537) between treatment groups.
No significant difference between groups in
biochemical markers of liver function except
INR (1.24 without NAC and 1.35 with NAC)
and creatinine (88.8 µmol/L without NAC and
72.6 µmol/L with NAC).
*There was significantly more delirium within
the NAC group (2.7% and 9.8%, P < 0.05) that
caused early trial termination.
Study had issues with randomization and
there were disproportional females in the
NAC group
no effect
potential safety
issue highlighted
Donadon 2016(124)
Italy
Double-blind
RCT
Hepatic
resection using
48
IV NAC 150 mg/kg as a
bolus for 1 hour before
surgery, then continuous
Morbidity was not significantly different
between the 3 groups.
Positive effect

47
Pringle
maneuver
infusion of 50 mg/kg/h
during the procedure
Compared to two other
regimens:
Methylprednisolone
(MET) 500 mg in 250 ml
glucose 5% as bolus 1 hr.
before surgery then
continuous infusion
OR
Placebo infusion (glucose
5%)
Comparing across the 3 groups, ALT at
postoperative day 1 was significantly lower in
both the MET and NAC groups vs placebo
(p=0.039), and significantly lower in the NAC
group as compared with the MET group
(p=0.021). At postoperative day 3, the 3-
group difference was not significant, but the
NAC group ALT was lower than the MET
group (p=0.041).
Similarly, the NAC group had lower bilirubin
as compared with the MET group (p=0.03) at
postop day 1; the 3 group levels were not
significantly different and no significant
differences at day 3 in bilirubin were
observed.
D’Amico, 2013(125)
Italy
RCT
First liver
transplant for
chronic liver
disease
patients
140
15 minute NAC infusion
(30 mg/kg) 1 hour before
the start of organ retrieval
and a locoregional
infusion (150 mg/kg of
estimated liver weight for
a maximum dose 300 mg)
in the portal vein just
before cross-clamping (69
patients)
71 patients assigned to
standard protocol without
NAC
The NAC harvesting protocol significantly
improves graft survival (graft survival rates at
3 and 12 months were 93% and 90%,
respectively, in the NAC group and 82% and
70%, respectively, in the control group (P =
0.02).
Post-op day 15 AST levels were 31 U/L (range
= 15‑150 U/L) in the study group and 38 U/L
(range = 13‑326 U/L) in the control group
(P = 0.02). No significant differences in any
other liver specific measures.
The incidence of postoperative complications
was lower in the NAC group (23%) versus the
control group (51%, P < 0.01).
Positive effect
Robinson
2013(126)
UK
Retrospective
cohort
Liver resection
88
IV NAC (10,000 mg/24 hr.
in 250 ml 5% dextrose)
starting at time of
parenchymal transaction
and continuing for 3 days
postoperatively or until a
fall in ALT in patients
operated on by one
surgeon
Comparison group:
patients resected by
NAC was associated with lower postoperative
ALT as compared with control (p=0.019); no
significant differences in serum bilirubin or
prothrombin time were observed between het
wo groups.
Grade A post-hepatectomy liver failure was
more common in the NAC group as compared
with controls (31.8% vs 9.1%, p=0.025);
incidence of no liver failure or grades B/C
post hepatectomy liver failure were not
significantly different between the NAC and
No effect

48
surgeons not using NAC
as part of their
perioperative regimen
control groups.
No statistically significant difference in
incidence of clinically important
complications between he two treatment
groups.
Regueira,
1997(127)
Spain
Retrospective
clinical study
Liver
transplant
62
Donor received IV of 6g of
NAC at least an hour
before extraction (25
patients)
In 37 transplants, the
donor did not receive any
specific treatment
Grafts that received NAC presented less
cytolysis and less elevation of transaminases
(AST maximum was 353.56 UI/L in NAC
group vs 825.97 UI/L in controls); (first day
post-transplant ALT 250.16 UI/L in NAC
group vs 440.54 UI/L in controls); bile
production and synthetic function were also
better.
Prothrombin time was significantly superior
in the first 5 days in the NAC group compared
to controls (P=.0062)
Patients in the NAC groups experienced acute
rejection significantly less than controls
(p=.0309)
Presumed positive
effect
Other operative procedures; not a complete list
El Hamamsy
2019(128)
Egypt
Double-blind,
placebo
controlled RCT
Patients
requiring ICU
admission and
total parenteral
nutrition for at
least 5 days
after colon
surgery
60
IV bolus NAC (100 mg/kg
dissolved in 5% dextrose)
infused over 15 minutes
then continuous infusion
50 mg/kg/day starting 1
hour before induction of
anesthesia and continuing
for 48 hours after the
operation
Comparison group
received infusion of
dextrose 5% at equal
volume and rate as the
NAC group
Most clinical and laboratory parameters did
not differ significantly between the two
groups, including MAP, heart rate,
temperature, respiratory rate, total leukocyte
count, platelets, serum creatinine, BUN, serum
sodium, serum potassium, TNF-α, and MDA.
Mean postoperative serum ALT was
significantly lower in the NAC-treated group
as compared with the control group (22.6±9.9
vs. 31.1±17.8, p=0.028).
Investigators note mild adverse effects of NAC
in 2 patients (6.6%), including hypotension
and tachycardia.
Positive effect
Onk, 2018(129)
Turkey
Prospective,
randomized
study
COPD patients
having
coronary artery
70
900 mg/day of NAC for 7
days until the day of
surgery (35 patients)
NAC pre-treatment has a stabilizing effect on
NH
3
and nitrogen metabolism in COPD
patients undergoing CABG (postoperative
24th hour, and postoperative 48th hour
Positive effect

49
bypass grafting
surgery
35 patients received
standard care only
(respectively, 39.0±8.8 vs. 55.4±19.6 and
40.1±8.4 vs. 53.2±20.2 mcg/dl).
Patients with COPD who were started on N-
acetylcysteine before surgery tend to have
lower lactic acid levels (1.7±0.9 vs. 2.1±1.2
and 1.2±0.5 vs. 1.8±1.4 mmol/L; p<0.01),
authors infer that this indicates a protective
effect of N-acetylcysteine against renal and
hepatic tissue damage
Serum AST, ALT, ALP levels demonstrated no
significant difference between the 2 groups at
any of the 4 measurement time points
(preoperative, during cardiopulmonary
bypass, 24
th
postoperative hour, and
48
th
postoperative hour) (p>0.05)
Belgaumkar,
2016(130)
UK
Single blinded,
RCT
Laparoscopic
sleeve
gastrectomy
20
NAC infusion at a
standard 150 mg/kg in
200 ml 5% dextrose over
15 min at induction of
anesthesia followed by an
infusion of 50 mg/kg in
500 ml of 5% dextrose
during surgical retraction
of the liver for a max of 4
hours (10 subjects)
10 control subjects
received standard
anesthetic medication
alone
NAC did not reduce intraoperative liver injury
No significant differences between liver
enzymes, rates of complications, length of
stay, white cell count, or liver injury scores:
The trial was not powered to detect
differences in clinical outcomes.
Authors comment: “The major drawback of
the study was a lack of a standardized,
reproducible toxic insult to the liver during
intraoperative liver retraction with the
Nathanson liver retractor. The pressure
applied to liver varied from patient to patient
depending on their body habitus,
intraabdominal dimensions and size and
texture of the liver. There are presently no
routine clinical methods of measuring tissue
oxygen tension or pressure within the liver.
This lack of uniformity lead the investigators
to conclude that a major study redesign would
be required to reach an robust conclusion.”
no effect
Septic shock

50
Rank, 2000(131)
Germany
Prospective,
double-blind,
placebo
controlled RCT
Septic shock
patients within
24 hours after
onset of sepsis
60
NAC infusion of 150
mg/kg in 250 mL of 5%
dextrose over a period of
15 mins, followed by a
continuous infusion of
12.5 mg/kg/hr. over 90
mins (30 patients)
(30 control patients
received 5% dextrose
only)
After NAC, a significant increase in absolute
liver blood flow index (2.7 vs. 3.3 L/min/m2;
p = .01) and cardiac index (5.0 vs. 5.7
L/min/m2; p = .02) was observed.
After NAC, microsomal liver function
improved, evidenced by a significant increase
in MEGX (p = .04). Liver blood flow index and
MEGX correlated significantly (r(s) = .57; p <
or = .01).
Positive effect

51
Appendix 3: Summary of adverse events reported in systematic reviews, by indication
Indication
Number of
participants
(studies)
Patient characteristics
Major adverse event(s) reported
Frequency
Acetaminophen
overdose(83)
1058 (8
prospective trials
of IV NAC)
Adult and pediatric
patients, males and
females
Anaphylactoid reactions including:
Up to 48% in prospective studies
Nausea and vomiting
From 4.5% to 70.4%
Cutaneous (rash, flushing, pruritis)
From 3.6% to 32%
Bronchospasm
From 4 to 18%
Hypotension
15.7% (only reported in one small study
with 27 patients)
Chest pain
6.5% (only reported in one small study of
11 patients)
Decrease in INR
One study(132) reported the effect in
100% of patients (of 87)
Acetaminophen
overdose(85)
6455 (1
retrospective
medical review of
patients treated
with IV NAC at 34
Canadian
hospitals)
Adult patients, males and
females
Anaphylactoid reactions including:
8.2%
Cutaneous only (rash, flushing, pruritis)
6.2%
Systemic only
.5%
Both cutaneous and systemic
1.5%
Respiratory effects and/or hypotension
2%
Acetaminophen
overdose(82)
503 (multi-
center,
retrospective
chart review of
patients treated
with oral or IV
NAC)
Adult patients, males and
females
No serious adverse events reported.
Nausea and vomiting were mid and the
most common.
23% oral versus 9% IV
Anaphylactoid reactions
2% oral versus 6% IV
Psychiatric and
neurological
disorders(93)
~1300 (16
controlled studies
of patients
treated with oral
and IV NAC)
Adult and pediatric
patients, males and
females
Largest rate of AEs was seen in an open-
label cannabis study (24 patients)(133)
63% reported at least one AE
Abdominal discomfort
20.8%
Muscle pains/aches
20.8%

52
Insomnia
16.6%
Headache, nasal congestion, nausea,
weight decrease, restlessness, or
dizziness
12.5%
Idiopathic
pulmonary
fibrosis(95)
1003 (12 RCTs of
patients treated
with oral or
inhaled NAC
reported safety)
Adult patients, males and
females
Most commonly reported AEs were
cough, dyspnea, bacterial pneumonia,
diarrhea, headache, edema, abdominal
pain
No trial reported a significantly increased
risk of adverse events among patients
treated with NAC.
Contrast
neuropathy(96)
1776 (15,
prospective, case-
controlled studies
of patients
treated with oral
and IV NAC)
Adult patients, males and
females
No major treatment-related adverse
events were reported.
No trial reported a significantly increased
risk of adverse events among patients
treated with NAC.
Chronic
bronchitis(134)
2,540 (11
randomized,
controlled trials
of patients
treated with oral
NAC)
Adult patients, males, and
females
6 trials reported GI adverse effects
No trial reported a significant increase in
adverse events among patients treated
with NAC.

53
Acute liver
failure not
caused by
acetaminophen
overdose(75)
170 (1
retrospective
case-controlled
medical record
review, IV NAC
administered to
111 compared to
59 who did not
receive NAC)
Pediatric patients, males
and females
Adverse effects included rash, cardiac
reactions which were not attributed to
drug administration (3 children), mild
dizziness and peripheral edema (1
child), and bronchospasm (1 child)
which was attributed to an allergic
reaction (NAC was immediately
stopped)
Adverse events were reported in 11% of
patients but there was not a significant
increase in adverse events among patients
treated with NAC.
Psychiatric
conditions(135)
765 (12
randomized,
controlled trials
of oral NAC)
Pediatric patients, males
and females
Mild adverse effects were reported
including constipation (16.1%),
increased appetite (16.1%), fatigue
(12.9%), nervousness (12.9%), daytime
sleepiness (12.9%) in ASD studies,
headache and aggressive behavior in
nail biting, and headache (Tourette’s
syndrome)
No trial reported a significantly increased
risk of adverse events among patients
treated with NAC.
