
Breast Cancer
Facts & Figures 2017-2018

Contents
Breast Cancer Basic Facts 1
Table 1. Estimated New Female Breast Cancer Cases
and Deaths by Age, US, 2017 1
Figure 1. Age-specific Female Breast Cancer Incidence Rates
by Race/Ethnicity, 2010-2014, US 2
Breast Cancer Occurrence 3
Table 2. Age-specific Probability of Developing Invasive
Breast Cancer for US Women 4
Figure 2. Female Breast Cancer Incidence (2010-2014)
and Mortality (2011-2015) Rates by Race/Ethnicity, US 4
Figure 3. Female Breast Cancer Incidence Rates
by Subtype and Race/Ethnicity, 2010-2014, US 5
Table 3. Female Breast Cancer Incidence (2010-2014) and
Mortality (2011-2015) Rates by Race/Ethnicity and State 6
Figure 4. Geographic Variation in Female Breast Cancer
Death Rates by Race, 2011-2015 7
Figure 5. Trends in Incidence Rates of Invasive and In Situ
Female Breast Cancer by Age, 1975-2014, US 8
Figure 6a. Trends in Female Breast Cancer Incidence
Rates by Race/Ethnicity, 1975-2014, US 9
Figure 6b. Trends in Female Breast Cancer Death
Rates by Race/Ethnicity, 1975-2015, US 9
Figure 7. Trends in Female Breast Cancer Incidence
Rates by Tumor Size, 1992-2014, US 9
Figure 8. Trends in Female Breast Cancer Incidence
Rates by Stage and Race/Ethnicity, 1992-2014, US 10
Figure 9. Female Breast Cancer-specific Survival and
Stage Distribution by Race/Ethnicity, 2007-2013, US 11
Figure 10. Trends in Female Breast Cancer 5-year
Relative Survival Rates by Race, 1975-2013, US 11
Breast Cancer Risk Factors 12
Table 4. Factors That Increase the Relative Risk for Breast Cancer in
Women 13
Breast Cancer Screening 19
Table 5. Prevalence of Mammography (%), Women
40 and Older, US, 2015 21
Table 6. Prevalence of Mammography* (%) by State,
Women 40 and Older, 2014 23
Breast Cancer Treatment 24
Figure 11. Female Breast Cancer Treatment Patterns (%),
by Stage, 2013, US 25
What Is the American Cancer Society
Doing About Breast Cancer? 28
Sources of Statistics 32
References 33
Global Headquarters: American Cancer Society Inc.
250 Williams Street, NW, Atlanta, GA 30303-1002
404-320-3333
©2017, American Cancer Society, Inc. All rights reserved,
including the right to reproduce this publication
or portions thereof in any form.
For written permission, address the Legal department of
the American Cancer Society, 250 Williams Street, NW,
Atlanta, GA 30303-1002.
This publication attempts to summarize current scientific information
about breast cancer. Except when specified, it does not represent
the official policy of the American Cancer Society.
Suggested citation: American Cancer Society. Breast Cancer Facts &
Figures 2017-2018. Atlanta: American Cancer Society, Inc. 2017.

Breast Cancer Facts & Figures 2017-2018 1
Breast Cancer Basic Facts
What is breast cancer?
Cancer is a group of diseases that cause cells in the body
to change and spread out of control. Most types of cancer
cells eventually form a lump or mass called a tumor, and
are named after the part of the body where the tumor
originates. Most breast cancers begin either in the breast
tissue made up of glands for milk production, called
lobules, or in the ducts that connect the lobules to the
nipple. The remainder of the breast is made up of fatty,
connective, and lymphatic tissues.
What are the signs and symptoms
of breast cancer?
Breast cancer typically produces no symptoms when the
tumor is small and most easily treated, which is why
screening is important for early detection. The most
common physical sign is a painless lump. Sometimes
breast cancer spreads to underarm lymph nodes and
causes a lump or swelling, even before the original breast
tumor is large enough to be felt. Less common signs and
symptoms include breast pain or heaviness; persistent
changes, such as swelling, thickening, or redness of the
skin; and nipple abnormalities such as spontaneous
discharge (especially if bloody), erosion, or retraction.
Any persistent change in the breast should be evaluated
by a physician as soon as possible.
How is breast cancer diagnosed?
Breast cancer is typically detected either during a
screening examination, before symptoms have developed,
or after a woman notices a lump. Most masses seen on a
mammogram and most breast lumps turn out to be
benign (not cancerous), do not grow uncontrollably or
spread, and are not life-threatening. When cancer is
suspected, microscopic analysis of breast tissue is
necessary for a diagnosis and to determine the extent of
spread (stage) and characterize the type of the disease.
The tissue for microscopic analysis can be obtained from
a needle biopsy (fine-needle or wider core needle) or
surgical incision. Selection of the type of biopsy is based
on multiple factors, including the size and location of the
mass, as well as patient factors and preferences and
resources.
How is breast cancer staged?
The prognosis of invasive breast cancer is strongly
influenced by the stage of the disease – that is, the extent
or spread of the cancer when it is first diagnosed. There
are two main staging systems for cancer. The TNM
classification of tumors uses information on tumor size
and how far it has spread within the breast and to adjacent
tissues (T), the extent of spread to the nearby lymph nodes
(N), and the presence or absence of distant metastases
(spread to distant organs) (M).
1
Once the T, N, and M are
determined, a stage of 0, I, II, III, or IV is assigned, with
stage 0 being in situ (abnormal cells have not penetrated
the ducts or glands from which they originated), stage I
being early-stage invasive cancer, and stage IV being the
most advanced disease. The TNM staging system is
commonly used in clinical settings. The latest revision
(8th edition) to the TNM stage for breast cancer also
incorporates biologic factors in order to further refine the
breast cancer staging system and will be implemented by
oncology programs in 2018.
2
Table 1. Estimated New Female Breast Cancer Cases
and Deaths by Age, US, 2017
In Situ Cases Invasive Cases Deaths
Age Number % Number % Number %
<40 1,610 3% 11,16 0 4% 990 2%
40-49 12,4 40 20% 36,920 15% 3,480 9%
50-59 17, 68 0 28% 58,620 23% 7,590 19%
60-69 17,550 28% 68,070 27% 9,420 23%
70-79 10,370 16% 47,860 19% 8,220 20%
80+ 3,760 6% 30,080 12% 10,910 27%
All ages 63,410 252,710 40,610
Estimates are rounded to the nearest 10. Percentages may not sum to 100
due to rounding.
©2017, American Cancer Society, Inc., Surveillance Research

2 Breast Cancer Facts & Figures 2017-2018
The Surveillance, Epidemiology, and End Results (SEER)
Summary Stage system is more simplified and is
commonly used in reporting cancer registry data and for
public health research and planning.
According to the SEER Summary Stage system:
• In situ stage refers to the presence of abnormal cells
that have not invaded nearby tissues (corresponding
to stage 0 in the TNM staging system).
• Local stage refers to cancers that are confined to the
breast (corresponding to stage I and some stage II
cancers).
• Regional stage refers to tumors that have spread to
surrounding tissue or nearby lymph nodes (generally
corresponding to stage II or III cancers, depending
on size and lymph node involvement).
• Distant stage refers to cancers that have
metastasized (spread) to distant organs or lymph
nodes above the collarbone (corresponding to some
stage IIIc and all stage IV cancers).
What are the types of breast cancer?
In Situ
There are two main types of in situ breast cancer: ductal
carcinoma in situ (DCIS) and lobular carcinoma in situ
(LCIS), also known as lobular neoplasia. Other in situ
breast cancers have characteristics of both ductal and
lobular carcinomas or have unknown origins.
• Ductal carcinoma in situ. DCIS (83% of in situ cases
diagnosed during 2010-2014) refers to a condition in
which abnormal cells replace the normal epithelial
cells that line the breast ducts and may greatly
expand the ducts and lobules. DCIS may or may not
progress to invasive cancer; in fact, sometimes DCIS
grows so slowly that even without treatment it would
not affect a woman’s health. Long-term studies of
women whose DCIS was untreated because it was
originally misclassified as benign found that 20%-
53% were diagnosed with an invasive breast cancer
over the course of 10 or more years.
3-7
• Lobular carcinoma in situ. LCIS (13% of in situ
cases) refers to abnormal cells growing within and
expanding some of the lobules of the breast. LCIS is
generally not thought to be a precursor of invasive
cancer, but is a strong risk factor for developing
invasive cancer.
See pages 13 and 24 for additional information on
DCIS and LCIS. More information can also be found in
the Cancer Facts & Figures 2015, Special Section: Breast
Carcinoma In Situ.
Invasive
Most (80%) breast cancers are invasive, or infiltrating,
which means they have broken through the walls of the
glands or ducts where they originated and grown into
surrounding breast tissue. Although breast cancer
generally has been referred to as a single disease, there
are up to 21 distinct histological subtypes and at least
four different molecular subtypes that differ in terms of
risk factors, presentation, response to treatment, and
outcomes.
8-10
Gene expression profiling techniques have
allowed better understanding of the molecular subtypes of
breast cancers; however, this is a costly and complicated
Figure 1. Age-specific Female Breast Cancer Incidence
Rates by Race/Ethnicity, 2010-2014, US
Rate per 100,000
Age
Note: Rates are per 100,000 and age adjusted to the 2000 US standard
population.
Sources: Incidence: North American Association of Central Cancer Registries
(NAACCR), 2017. Mortality: National Center for Health Statistics, Centers for
Disease Control and Prevention, 2017.
American Cancer Society, Inc., Surveillance Research, 2017
Non-Hispanic White
Non-Hispanic Black
Hispanic
Asian/Pacific Islander
0
100
200
300
400
500
85+80-8475-7970-7465-6960-6455-5950-5445-4940-4435-3930-3425-2920-24

Breast Cancer Facts & Figures 2017-2018 3
process and is not currently standard practice.
Approximations of molecular subtypes have been
identified using routinely evaluated biological markers,
including the presence or absence of hormone (estrogen
or progesterone) receptors (HR+/HR-) and excess levels of
human epidermal growth factor receptor 2 (HER2, a
growth-promoting protein) and/or extra copies of the
HER2 gene (HER2+/HER2-).
11
The four main molecular
subtypes and their distribution are described here.
• Luminal A (HR+/HER2-) (71%). These cancers tend
to be slow-growing and less aggressive than other
subtypes. Luminal A tumors are associated with the
most favorable prognosis, particularly in the short
term, in part because they are more responsive to
anti-hormone therapy (see page 27).
12, 13
• Triple negative (HR-/HER2-) (12%). So called
because they are estrogen receptor (ER)-,
progesterone receptor (PR)-, and HER2-, these
cancers are twice as common in black women as
white women in the US, and are also more common
in premenopausal women and those with a BRCA1
gene mutation.
14
The majority (about 75%) of triple
negative breast cancers fall in to the basal-like
subtype defined by gene expression profiling. Triple
negative breast cancers have a poorer short-term
prognosis than other subtypes, in part because there
are currently no targeted therapies for these tumors.
15
• Luminal B (HR+/HER2+) (12%). Like luminal A
cancers, luminal B cancers are ER+ and/or PR+ and
are further defined by being highly positive for Ki67
(indicator of a large proportion of actively dividing
cells) or HER2. Luminal B breast cancers tend to be
higher grade and are associated with poorer survival
than luminal A cancers.
13
• HER2-enriched (HR-/HER2+) (5%). HER2-enriched
cancers tend to grow and spread more aggressively
than other subtypes and are associated with poorer
short-term prognosis compared to HR+ breast
cancers.
13
However, the recent widespread use of
targeted therapies for HER2+ cancers has improved
outcomes for these patients. For more information
about the treatment of HER2+ breast cancers, see the
section on targeted therapy on page 28.
Breast Cancer Occurrence
How many cases and deaths are
estimated to occur in 2017?
In 2017, an estimated 252,710 new cases of invasive breast
cancer will be diagnosed among women (Table 1, page 1)
and 2,470 cases will be diagnosed in men. In addition,
63,410 cases of in situ breast carcinoma will be diagnosed
among women. Approximately 40,610 women and 460
men are expected to die from breast cancer in 2017.
How many women alive today have
ever had breast cancer?
More than 3.5 million US women with a history of breast
cancer were alive on January 1, 2016.
16
Some of these
women were cancer-free, while others still had evidence
of cancer and may have been undergoing treatment.
Who gets breast cancer?
Age
• Breast cancer incidence and death rates generally
increase with age (Figure 1). The decrease in
incidence rates that occurs in women 80 years of age
and older may reflect lower rates of screening, the
detection of cancers by mammography before 80
years of age, and/or incomplete detection.
• During 2010-2014, the median age at the time of
breast cancer diagnosis was 62.
17
This means that
half of women who developed breast cancer were 62
years of age or younger at the time of diagnosis. The
median age of diagnosis is younger for black women
(59) than white women (63).
17

4 Breast Cancer Facts & Figures 2017-2018
• A woman living in the US has a 12.4%, or a 1-in-8,
lifetime risk of being diagnosed with breast cancer
(Table 2). Conversely, 7 out of 8 women born today will
not be diagnosed with breast cancer in their lifetimes.
In the 1970s, the lifetime risk of being diagnosed with
breast cancer was 1 in 11. This increase in risk over
the past four decades is due to longer life expectancy,
as well as increases in breast cancer incidence due in
part to changes in reproductive patterns, menopausal
hormone use, the rising prevalence of obesity, and
increased detection through screening. Lifetime risk
reflects an average woman’s risk over an entire
lifetime, including the possibility that she may die
from another cause before she would have been
diagnosed with breast cancer and does not apply only
to women who live to a very old age.
Race/Ethnicity
• Figure 2 shows breast cancer incidence and death
rates by race and ethnicity during the most recent
time period. Incidence and death rates for breast
cancer are higher among non-Hispanic white (NHW)
and non-Hispanic black (NHB) women than other
racial and ethnic groups. Asian/Pacific Islander (API)
women have the lowest incidence and death rates.
• Between the ages of 65 and 84, NHW women have
markedly higher breast cancer incidence rates than
NHB women (Figure 1, page 2). However, NHB
women have higher incidence rates before age 40 and
are more likely to die from breast cancer at every age.
• Racial/ethnic variation in incidence rates for specific
breast cancer subtypes are shown in Figure 3. NHW
women have the highest rates of HR+/HER2- breast
cancers, whereas NHB women have the highest rates
of triple negative breast cancers.
Are there geographic differences in
breast cancer rates?
Table 3, page 6 shows the variation in state-level
breast cancer incidence and death rates per 100,000
women by race/ethnicity. Although the overall incidence
rate for breast cancer in the US remains slightly higher in
NHW women compared to NHB women, in 9 of 43 states
with data for both groups, rates are higher among NHB
women. Data for AI/AN women are too sparse to provide
by state; however, a recent study found that rates were
more than 2-fold higher among women in Alaska (141.3
per 100,000) than those living in the Southwest US (59.6
per 100,000) during 1999-2009.
18
Table 2. Age-specific Probability of Developing Invasive
Breast Cancer for US Women
Current age 10-year probability: or 1 in:
20 0.1% 1,567
30 0.5% 220
40 1.5% 68
50 2.3% 43
60 3.4% 29
70 3.9% 25
Lifetime risk 12.4% 8
Note: Probability is among those free of cancer at beginning of age interval.
Based on cases diagnosed 2012-2014. Percentages and “1 in” numbers may
not be numerically equivalent due to rounding.
©2017, American Cancer Society, Inc., Surveillance Research
*Statsitics based on data from Contract Health Service Delivery Area (CHSDA)
counties. Note: Rates are age adjusted to the 2000 US standard population.
Sources: Incidence – NAACCR, 2017. Mortality – National Center for Health
Statistics, Centers for Disease Control and Prevention, 2017.
©2017, American Cancer Society, Inc., Surveillance Research
Rate per 100,000
Incidence Mortality
0
30
60
90
120
150
Asian/
Pacific Islander
Hispanic/
Latina
American Indian/
Alaska Native*
Non-Hispanic
Black
Non-Hispanic
White
20.8
29.5
14.3
14.2
11.3
128.7
125.5
100.7
91.9
90.7
Figure 2. Female Breast Cancer Incidence (2010-2014)
and Mortality (2011-2015) Rates by Race/Ethnicity, US

Breast Cancer Facts & Figures 2017-2018 5
In contrast to incidence, breast cancer death rates are
higher among NHB women than NHW women in every
state, with rates in some states (e.g., Louisiana and
Mississippi) as much as 60% higher. Death rates reflect
both cancer incidence and survival. Breast cancer
mortality rates among white women tend to be highest in
the North Central, Mid-Atlantic, and Western regions of
the US. Among black women, the highest death rates are
found in some of the South Central and Mid-Atlantic
states, as well as California (Figure 4, page 7). Factors
that contribute to geographic disparities include
variations in risk factors and access to screening and
treatment, which are influenced by socioeconomic
factors, legislative policies, and proximity to medical
services.
How has the occurrence of breast
cancer changed over time?
Incidence trends
Figure 5, page 8 presents trends for in situ and
invasive breast cancer incidence rates since 1975, when
population-based cancer registration began in the 9
oldest Surveillance, Epidemiology and End Results
(SEER) registries.
Incidence rates of in situ and invasive breast cancer rose
rapidly during the 1980s and 1990s (Figure 5a, page
8), largely because of increases in mammography
screening. The widespread uptake of mammography
screening inflated the incidence rate because cancers
were being diagnosed 1 to 3 years earlier than they would
have been in the absence of screening, and may also have
led to the detection of indolent (very slow-growing)
tumors. In addition, some of the historic increase in
breast cancer incidence reflects changes in reproductive
patterns, such as delayed childbearing and having fewer
children, which are known risk factors for breast cancer.
The increase in incidence was greater in women 50 years
of age and older than in those younger than 50.
Invasive breast cancer rates stabilized between 1987 and
1994 (Figure 5b, page 8). Incidence rates increased
again in the latter half of the 1990s, which may reflect
further increases in the prevalence of mammography
screening, as well as rising rates of obesity and the use of
menopausal hormones, both of which increase breast
cancer risk. Between 2002 and 2003, invasive breast
cancer rates dropped sharply (nearly 7%), likely due to
the decreased use of menopausal hormones following the
2002 publication of clinical trial results that found higher
risk of breast cancer and heart disease among users.
19, 20
The decline in incidence occurred primarily in white
women, in those 50 years of age and older, and for ER+
disease.
19, 21
From 2005 to 2014, the overall invasive breast
cancer incidence rate was stable, but the trends vary by
race and age.
Incidence rates of in situ breast cancer have been stable
since 2000 among women 50 and older and since 2007
among younger women.
HR = hormone receptor, HER2 = human epidermal growth factor receptor 2.
Note: Rates are age adjusted to the 2000 US standard population.
Source: NAACCR, 2017.
©2017, American Cancer Society, Inc., Surveillance Research
Rate per 100,000
Non-Hispanic White
Non-Hispanic Black
American Indian/Alaska Native
Hispanic/Latina
Asian/Pacific Islander
82
53
65
57
62
14 14
1212
11
5
7
4
6
5
12
10
10
8
Figure 3. Female Breast Cancer Incidence Rates by
Subtype and Race/Ethnicity, 2010-2014, US
0
20
40
60
80
100
Triple negativeHR-/HER2+HR+/HER2+HR+/HER2-
24

6 Breast Cancer Facts & Figures 2017-2018
Table 3. Female Breast Cancer Incidence (2010-2014) and Mortality (2011-2015) Rates by Race/Ethnicity and State
Incidence Mortality
State
Non-
Hispanic
White
Non-
Hispanic
Black
Hispanic/
Latina
Asian/
Pacific
Islander
Non-
Hispanic
White
Non-
Hispanic
Black
Hispanic/
Latina
Asian/
Pacific
Islander
Alabama 118.2 124.9 64.3 87.4 20.0 28.5 * *
Alaska 125.9 133.7 80.4 92.3 20.0 * * *
Arizona 120.5 107. 8 88.9 75.6 20.3 26.2 15.0 12.0
Arkansas 110.5 114. 2 148.1 126.9 20.5 30.3 15.3 *
California 139.0 129.0 89.2 95.7 23.1 31.9 14.5 12.6
Colorado 127.1 119.1 104.2 76.1 19.3 25.5 17.2 7.7
Connecticut 143.3 122.9 127.5 89.2 18.9 20.8 10.3 9.0
Delaware 135.8 130.6 100.0 85.9 21.2 26.0 * *
District of Columbia 157.6 141.6 70.8 90.4 22.6 34.4 * *
Florida 121.3 108.3 98 .1 70.1 20.1 25.8 15.3 9.8
Georgia 125.2 126.3 93.2 74.4 20.1 29.2 10.9 8.8
Hawaii 143.3 120.5 151.6 132.1 21.4 * * 14.4
Idaho 121.9 * 89.1 76.0 21.3 * * *
Illinois 135.7 131.8 89.5 92.7 22.5 31.2 10.9 11.7
Indiana 120.9 130.6 83.7 66.1 21.0 29.6 14.9 *
Iowa 124.7 105.1 6 7.1 68.2 19.3 21.4 * *
Kansas 124.4 126.2 84.2 63.2 19.9 30.1 11.6 *
Kentucky 124.3 127.0 54.2 64.4 21.4 28.5 * *
Louisiana 121.8 132.5 91.5 62.6 20.2 33.6 9.6 *
Maine 126.5 * * 76.3 18.0 * * *
Maryland 135.2 132.4 91.8 84.3 21.2 28.1 10.9 9.2
Massachusetts 141.8 114.7 87. 2 87.7 19.1 20.6 10.9 7.2
Michigan 122.0 127.1 80.0 85.3 21.0 29.8 16.2 9.2
Minnesota
†
131.5 103.1 103.1 68.9 18.8 23.0 11. 4 7.3
Mississippi 113.4 121.0 41.4 60.6 19.5 31.5 * *
Missouri 126.0 133.4 78.9 88.2 21.3 32.6 10.5 14.4
Montana 122.6 * 134.3 112.5 20.4 * * *
Nebraska 123.7 127.8 93.4 65.6 20.3 27.8 * *
Nevada
†
121.3 108.1 75.5 78.5 24.5 29.4 11. 2 14.4
New Hampshire 142.3 * 92.2 72.1 20.2 * * *
New Jersey 142.3 125.9 98.7 92.2 23.0 32.3 13.2 11. 2
New Mexico
†
123.2 98.8 103.2 65.6 21.2 32.4 16.8 *
New York 139.6 119.5 101.3 92.2 20.2 26.8 14.7 9.6
North Carolina 130.3 134.1 82.5 77.7 19.7 29.1 10.1 12.1
North Dakota 122.2 * * * 17.5 * * *
Ohio 123.7 123.8 64.3 79.8 22.2 31.0 9.9 10.9
Oklahoma 114. 8 122.9 96.9 80.5 23.0 33.6 13.1 *
Oregon 128.5 127.1 97.0 76.9 20.8 30.0 12.1 9.9
Pennsylvania 131.8 130.8 86.5 73.8 21.2 31.7 12.2 11.2
Rhode Island 135.6 113. 4 85.2 68.9 18.8 26.9 * *
South Carolina 128.8 125.7 89.6 76.3 20.5 29.0 9.4 *
South Dakota 132.1 * * * 20.2 * * *
Tennessee 121.8 126.3 66.5 70.2 20.7 31.5 11.5 11.6
Texas 122.5 120.3 88.0 65.0 20.6 30.4 15.5 9.9
Utah 116. 8 90.3 101.8 91.2 20.9 * 11.8 19.2
Vermont 130.5 * * * 19.0 * * *
Virginia 130.0 134.3 80.6 79.0 21.0 29.5 11.7 9.8
Washington 139.3 125.7 93.6 98.3 20.9 25.6 9.7 11.1
West Virginia 115.1 120.1 * 77. 8 22.2 30.5 * *
Wisconsin 129.1 133.5 90.2 74.2 19.8 31.6 7.7 *
Wyoming 116.1 * 85.5 * 19.4 * * *
United States 128.7 125.5 91.9 90.7 20.8 29.5 14.2 11.3
Note: Rates are per 100,000 and age adjusted to 2000 US standard population. *Statistic not displayed due to fewer than 25 cases or deaths. †This state’s registry did
not achieve high-quality data standards for one or more years during 2010-2014, according to NAACCR data quality indicators and are not included in the overall US
incidence rate.
Sources: Incidence: NAACCR, 2017. Mortality: National Center for Health Statistics, Centers for Disease Control and Prevention, 2017.
©2017, American Cancer Society, Inc., Surveillance Research
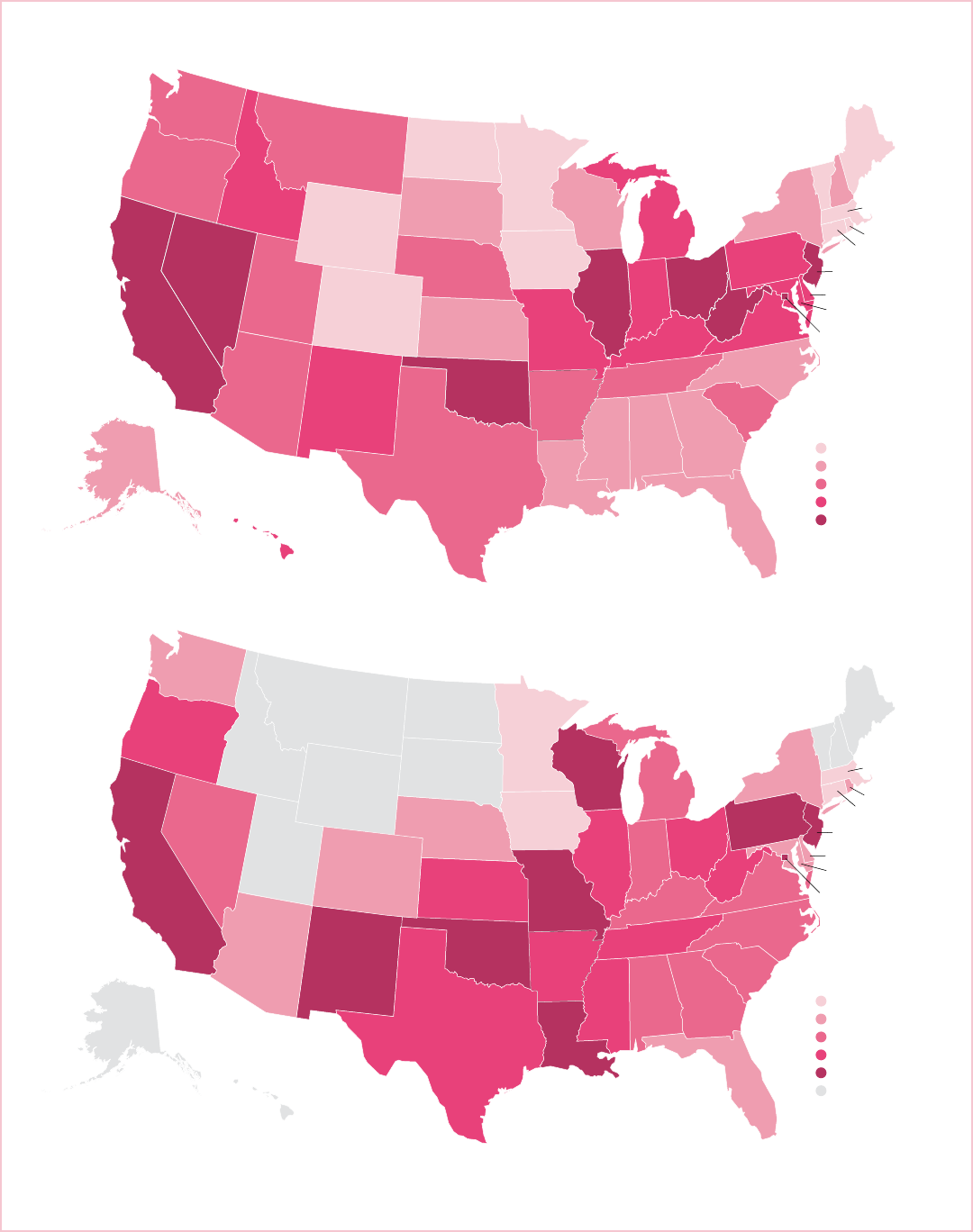
Breast Cancer Facts & Figures 2017-2018 7
AL
AZ
AR
CO
CT
DE
FL
GA
ID
IN
IA
KS
KY
LA
ME
MD
MA
MN
MS
MO
MT
NE
NH
NJ
NM
NY
NC
ND
OR
PA
RI
SC
SD
TN
TX
UT
VT
VA
WA
WI
WY
DC
AK
HI
MI
Note: Rates are per 100,000 and age adjusted to the 2000 US standard population. Statistic not displayed for states with fewer than 25 deaths during 2011-2015.
Source: National Center for Health Statistics, Centers for Disease Control and Prevention, 2017.
©2017, American Cancer Society, Inc., Surveillance Research
Deaths per 100,000
17.5 - 19.4
19.5 - 20.2
20.3 - 20.9
21.0 - 21.4
21.5 - 24.5
Deaths per 100,000
20.6 - 23.0
23.1 - 28.1
28.2 - 29.8
29.9 - 31.5
31.6 - 34.4
Data not shown
AL
AZ
AR
CA
CO
CT
DE
FL
GA
ID
IL
IN
IA
KS
KY
LA
ME
MD
MA
MN
MS
MO
MT
NE
NV
NH
NJ
NM
NY
NC
ND
OH
OK
OR
PA
RI
SC
SD
TN
TX
UT
VT
VA
WA
WV
WI
WY
DC
AK
HI
MI
Non-Hispanic Blacks
Non-Hispanic Whites
Figure 4. Geographic Variation in Female Breast Cancer Death Rates by Race, 2011-2015
OH
WV
IL
OK
CA
NV

8 Breast Cancer Facts & Figures 2017-2018
Race/Ethnicity: Figure 6a presents trends in invasive
female breast cancer incidence rates by race and
ethnicity. Incidence data are available for white and black
women since 1975 and for women of other races and
ethnicities since 1992. During 2005-2014, overall breast
cancer incidence rates increased among API (1.7% per
year), NHB (0.4% per year), and Hispanic (0.3% per year)
women, but were stable among NHW and AI/AN women.
22
Age: Trends for invasive breast cancer by age at diagnosis
are shown in Figure 5b. Although long-term data (shown)
suggest breast cancer incidence rates have increased
slightly among women over the age of 50 during the
most recent period (2005-2014), data with broader
coverage indicate that rates are relatively stable in this
age group.
22
In contrast, among women under age 50,
incidence rates have slowly increased (0.2% per year)
since the mid-1990s.
22
Tumor size: Incidence rates during 2005-2014 were stable
for smaller (≤ 2.0 cm) tumors and increased by 1.3%
annually for 2.1-5.0 cm tumors and 1.9% annually for
tumors larger than 5.0 cm (Figure 7).
Stage: Incidence rates during 2005-2014 increased for
localized breast cancer among NHW (0.7% per year),
NHB (1.5%), API (2.1%), and Hispanic (0.6%) women;
decreased (NHWs, 1.4% per year) or remained stable for
regional stage tumors; and increased for distant stage
tumors for all groups (NHWs: 2.0% per year; API: 1.7%;
Hispanics: 0.7%) except NHBs (Figure 8, page 10).
22
Incidence rates for breast cancer with unknown stage
decreased in all groups. The decline for regional stage
disease in NHWs may reflect a shift toward earlier stage
at diagnosis. The increase in distant-stage disease
coupled with the decrease in unknown stage may be due
to more complete staging of advanced tumors.
Mortality trends
Overall breast cancer death rates increased by 0.4% per
year from 1975 to 1989, but since have decreased rapidly,
for a total decline of 39% through 2015. As a result,
322,600 breast cancer deaths have been averted in US
women through 2015. The decrease occurred in both
younger and older women, but has slowed among women
younger than 50 since 2007. From 2006 through 2015,
breast cancer death rates declined annually by 2.6% in
Figure 5. Trends in Incidence Rates of Invasive and In Situ Female Breast Cancer by Age, 1975-2014, US
a. In Situ
Year
Rate per 100,000
b. Invasive
Year
Rate per 100,000
Note: Rates are age adjusted to the 2000 US standard population. Invasive breast cancer rates are adjusted for reporting delay.
Source: Surveillance, Epidemiology, and End Results (SEER) Program, SEER 9 Registries, National Cancer Institute, 2017.
American Cancer Society, Inc., Surveillance Research, 2017
0
50
100
150
200
250
300
350
400
201420102005200019951990198519801975
0
20
40
60
80
100
120
201420102005200019951990198519801975
Ages 20+
Ages 20-49
Ages 50+

Breast Cancer Facts & Figures 2017-2018 9
AI/ANs, 1.8% in NHWs, 1.5% in NHBs, 1.4% in Hispanics,
and 0.9% in APIs.
17
Notably, the decline among AI/AN
women began in 2005, more than a decade later than
other racial and ethnic groups.
The decline in breast cancer mortality has been
attributed to both improvements in treatment and early
detection.
23
However, not all women have benefited
equally, as indicated by the striking divergence in
mortality trends between black and white women
beginning in the early 1980s (Figure 6b). This disparity
likely reflects a combination of factors, including
differences in stage at diagnosis, obesity and comorbidities,
and tumor characteristics, as well as access, adherence,
and response to treatment.
24-27
It may also reflect
differences in mammography screening. Although
findings from national surveys indicate current
screening rates are similar between black and white
women, these estimates likely overestimate
mammography rates, especially for blacks.
28-30
As
treatment for breast cancers has improved, the racial
disparity widened; in 2015, breast cancer death rates
were 39% higher in black than white women.
Figure 6a. Trends in Female Breast Cancer Incidence
Rates by Race/Ethnicity, 1975-2014, US
Rate per 100,000
Year
Figure 6b. Trends in Female Breast Cancer Death Rates
by Race/Ethnicity, 1975-2015, US
Rate per 100,000
Year
Black
White
Hispanic/
Latina
Asian/Pacific Islander
White
Black
Asian/Pacific Islander
Hispanic/Latina
0
20
40
60
80
100
120
140
160
201420102005200019951990198519801975
0
5
10
15
20
25
30
35
40
45
201520102005200019951990198519801975
Note: Rates are age adjusted to the 2000 US standard population and adjusted
for reporting delays.
Source: SEER Program, National Cancer Institute, 2017. Data for whites and
blacks are from the 9 SEER registries and data for other races/ethnicities are
3-year moving averages from the 13 SEER registries. For Hispanics, incidence
data do not include cases from the Alaska Native Registry. Data for AI/AN not
shown due to small counts and unstable rates.
Note: Rates are age adjusted to the 2000 US standard population.
Source: National Center for Health Statistics, Centers for Disease Control and
Prevention, 2017. Rates for Hispanics exclude deaths from Louisiana, New
Hampshire, and Oklahoma. Data for AI/AN not shown due to small counts and
unstable rates.
American Cancer Society, Inc., Surveillance Research, 2017
Figure 7. Trends in Female Breast Cancer Incidence
Rates by Tumor Size, 1992-2014, US
Rate per 100,000
<2.0 cm
2.0-4.9 cm
5+ cm
unknown
Year
Note: Rates are age adjusted to the 2000 US standard population and adjusted
for reporting delays.
Source: 13 SEER Registries, National Cancer Institute, 2017.
American Cancer Society, Inc., Surveillance Research, 2017
0
10
20
30
40
50
60
70
80
201420122010200820062004200220001998199619941992

10 Breast Cancer Facts & Figures 2017-2018
Breast cancer survival
Relative survival rates are an estimate of the percentage
of patients who will survive for a given period of time
after a cancer diagnosis, accounting for normal life
expectancy. Survival among cancer patients is compared
to survival among people of the same age and race who
have not been diagnosed with cancer.
Based on the most recent data, relative survival rates for
women diagnosed with breast cancer are:
• 91% at 5 years after diagnosis
• 86% after 10 years
• 80% after 15 years
Relative survival rates should be interpreted with
caution. First, they are based on the average experience
of all women and do not predict individual prognosis
because many patient and tumor characteristics that
influence breast cancer survival are not taken into
account. Second, long-term survival rates are based on
the experience of women diagnosed and treated many
years ago and do not reflect the most recent
improvements in early detection and treatment.
Stage at diagnosis
Breast cancer survival varies by stage at diagnosis
(Figure 9a). The overall 5-year relative survival rate is
99% for localized disease, 85% for regional disease, and
27% for distant-stage disease.
17
Survival within each
stage varies by tumor size. For example, among women
with regional disease, the 5-year relative survival is 95%
for tumors less than or equal to 2.0 cm, 85% for tumors
2.1-5.0 cm, and 72% for tumors greater than 5.0 cm.
31
Race/ethnicity and socioeconomic factors
Since 1975, the breast cancer 5-year relative survival rate
has increased significantly for both black and white
women (Figure 10). While there remains a substantial
gap, especially for late-stage diagnoses, the racial
disparity seems to be narrowing. In the most recent
period, the 5-year relative survival rate was 83% for black
women and 92% for white women. The racial disparity in
survival reflects later stage at diagnosis and poorer
stage-specific survival in black women as well as higher
rates of more aggressive, triple negative breast cancer.
Cause-specific survival instead of relative survival is
used to describe the cancer experience of racial and
ethnic minorities because reliable life expectancy is not
available for some groups. Cause-specific survival is the
probability of not dying of breast cancer within five years
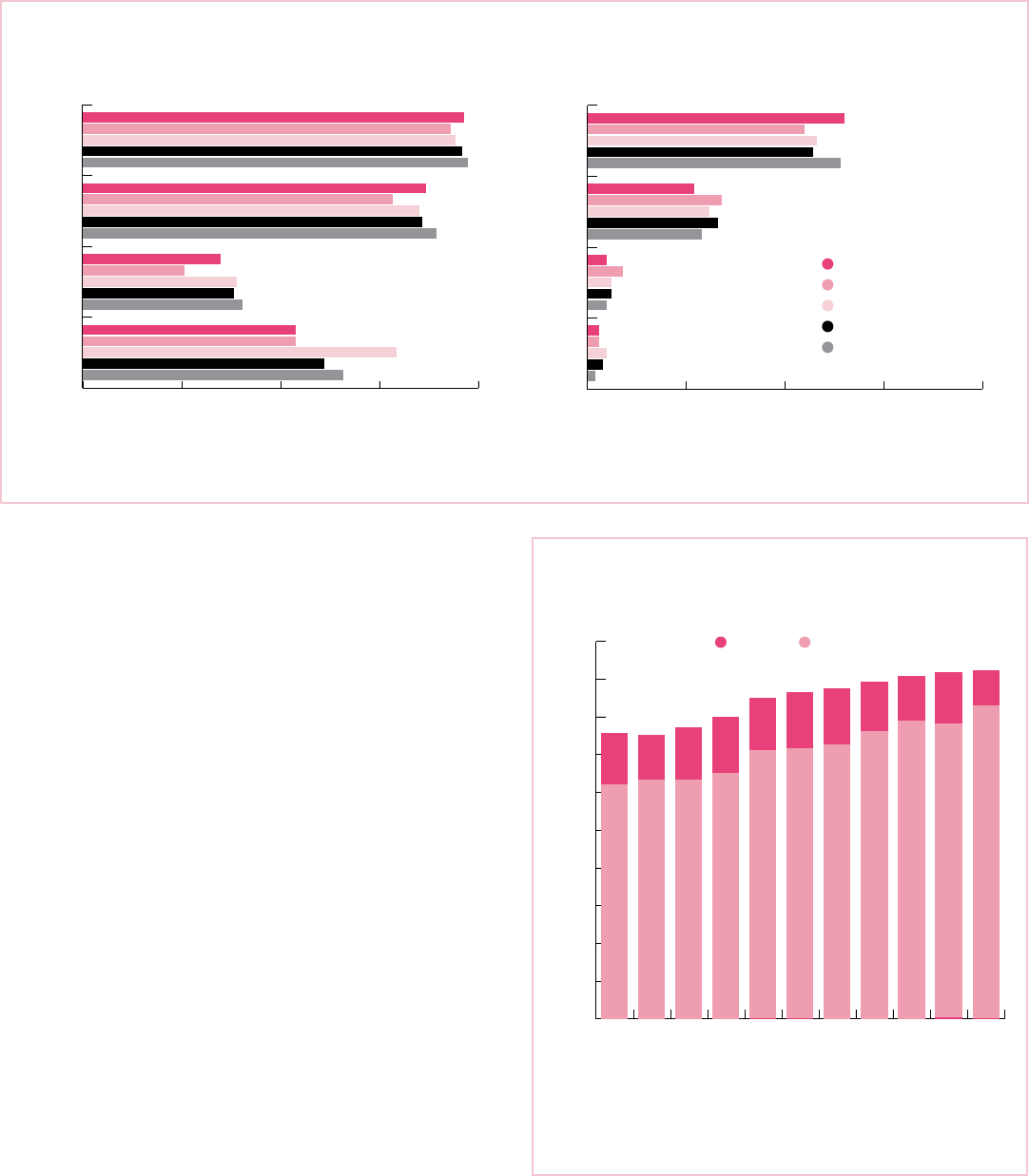
of diagnosis. For every stage of disease, API women have
the highest survival and NHB women have the lowest
survival (Figure 9). Poverty, less education, and a lack of
health insurance are associated with lower breast cancer
survival.
32-36
Figure 8. Trends in Female Breast Cancer Incidence
Rates by Stage and Race/Ethnicity, 1992-2014, US
Rate per 100,000
Non-Hispanic White Non-Hispanic Black
Rate per 100,000
Asian/Pacific Islander Hispanic
Note: Rates are two year moving averages, age adjusted to the 2000 US
standard population, and adjusted for reporting delay.
Source: 13 SEER Registries, National Cancer Institute, 2017.
American Cancer Society, Inc., Surveillance Research, 2017
0
10
20
30
40
50
60
70
80
90
100
20132008200319981993
0
10
20
30
40
50
60
70
80
90
100
20132008200319981993
0
10
20
30
40
50
60
70
80
90
100
20132008200319981993
0
10
20
30
40
50
60
70
80
90
100
20132008200319981993
Localized
Regional Distant Unknown
Breast Cancer Facts & Figures 2017-2018 11
Male breast cancer
Breast cancer in men is rare, accounting for less than 1%
of breast cancer cases in the US. However, since 1975, the
incidence rate has increased slightly, from 1.0 case per
100,000 men during 1975-1979 to 1.3 cases per 100,000
men during 2010-2014. Men are more likely than women
to be diagnosed with advanced-stage breast cancer,
which likely reflects decreased awareness and delayed
detection because screening mammography is not
recommended for men due to the rarity of the disease.
37
Similar to female breast cancer, the incidence of male
breast cancer increases with age. The death rate for male
breast cancer has decreased slightly from 0.4 (per
100,000) during 1975-1979 to 0.3 (per 100,000) during
2011-2015 due to improvements in treatment.
Due to the infrequency of male breast cancer, much less
is known about the disease than female breast cancer.
Risk factors include radiation exposure, BRCA 1/2 gene
mutations, Klinefelter syndrome, testicular disorders,
family history of breast or ovarian cancer, diabetes,
gynecomastia (enlarged breasts), and obesity.
38, 39
Figure 10. Trends in Female Breast Cancer 5-year
Relative Survival Rates by Race, 1975-2013, US
Percent
0
10
20
30
40
50
60
70
80
90
100
2007-
2013
2003-
2006
1999-
2001
1996-
1998
1993-
1995
1990-
1992
1987-
1989
1984-
1986
1981-
1983
1978-
1980
1975-
1977
Year
Survival rates are based on follow-up of patients through 2014.
Source: Howlader et al.
17
American Cancer Society, Inc., Surveillance Research, 2017
White Black
Figure 9. Female Breast Cancer-specific Survival and Stage Distribution by Race/Ethnicity, 2007-2013, US
a. Five-year Breast Cancer-specific Survival Rates (%) b. Stage Distribution (%)
0 25 50 75 100
Unstaged
Distant
Regional
Localized
0 25 50 75 100
Unstaged
Distant
Regional
Localized
93
97
98
94
96
78
87
90
85
86
26
35
40
39
38
54
54
66
80
61
Survival rates are based on patients diagnosed during 2007-2013 and followed through 2014. Stage distribution percentages may not sum to 100 due to rounding.
Sources: Survival – SEER Program, 18 SEER registries, National Cancer Institute, 2016. Stage distribution – NAACCR, 2017.
American Cancer Society, Inc., Surveillance Research, 2017
Non-Hispanic White
Non-Hispanic Black
American Indian/Alsaka Native
Hispanic
Asian/Pacific Islander
55
64
65
58
57
34
27
29
31
33
9
5
6
6
3
3
2
5
5
4

12 Breast Cancer Facts & Figures 2017-2018
Breast Cancer Risk Factors
About one-third of postmenopausal breast cancers are
thought to be caused by behavioral factors that are
modifiable, such as postmenopausal obesity, physical
inactivity, use of combined estrogen and progestin
menopausal hormones, alcohol consumption, and not
breastfeeding.
40
Many risk factors affect lifetime
exposure of breast tissue to hormones (early menarche,
late menopause, obesity, and hormone use). Hormones
are thought to influence breast cancer risk by increasing
cell proliferation, thereby increasing the likelihood of
DNA damage, as well as promoting cancer growth.
Although exposures that influence risk accumulate
throughout a woman’s life, research suggests that the
time between menarche and first pregnancy may be
particularly critical.
41, 42
Many established risk factors for
breast cancer are specifically associated with HR+/
luminal breast cancer; less is known about risk factors
for HR-, HER2+ or basal-like breast cancers.
43
Factors
associated with an increased or decreased risk of breast
cancer are discussed below.
Family history and personal
characteristics
Family history
Women and men with a family history of breast cancer,
especially in a first-degree relative (parent, child, or
sibling), are at increased risk for the disease. Compared
to women without a family history, risk of breast cancer
is about 2 times higher for women with one affected
first-degree female relative and 3-4 times higher for
women with more than one first-degree relative.
44
Risk is further increased when the affected relative was
diagnosed at a young age or if the cancer was diagnosed
in both breasts. It is important to note that the majority
of women with one or more affected first-degree relatives
will never develop breast cancer and that most women
who develop breast cancer do not have a family history
of the disease.
A family history of ovarian cancer is also associated with
increased breast cancer risk in both men and women.
Women with a family history of breast or ovarian cancer
should discuss this with their physician or a genetic
counselor because it may signal the presence of a genetic
predisposition to cancer.
Genetic predisposition
Inherited mutations (genetic alterations) in BRCA1 and
BRCA2, the most well-studied breast cancer susceptibility
genes, account for 5%-10% of all female breast cancers,
5%-20% of male breast cancer, and 15%-20% of all familial
breast cancers.
45, 46
These mutations are rare (much less
than 1%) in the general population, but occur slightly
more often in certain ethnic or geographically isolated
groups, such as those of Ashkenazi (Eastern European)
Jewish descent (about 2%).
47
Compared to women in the
general population who have a 10% risk of developing
breast cancer by 80 years of age, the corresponding risks
for BRCA1 and BRCA2 mutation carriers are estimated to
be as much as 70%.
48
Mutations in PALB2, a different gene
that works with BRCA2, appear to confer risk that may be
as high as BRCA2 mutations.
49
Other inherited conditions associated with a smaller
increase in breast cancer risk include the Li-Fraumeni
and Cowden syndromes.
45
In addition, more than 150 less
rare genetic variants are associated with slightly elevated
risk.
50
Scientists now believe that much of the occurrence
of breast cancer clustered in families results from the
interaction between lifestyle factors and these low-risk
variations.
51
The US Preventive Services Task Force recommends
primary care providers routinely collect and update
family medical history and screen women with a family
history of breast, ovarian, tubal, or peritoneal cancer
with one of several brief questionnaires to determine if
there is a need for in-depth genetic counseling to consider
BRCA testing.
52
Those who consider testing are strongly
encouraged to talk with a genetic counselor before
making a decision so that the benefits and potential
consequences can be understood and carefully considered.

Breast Cancer Facts & Figures 2017-2018 13
Personal history of breast cancer
Women diagnosed with breast cancer have a small
increased risk of developing a new cancer in the opposite
breast; however, rates of second breast cancers have
declined steadily since 1985.
53
The decrease has
predominantly been among ER+ breast cancer patients
and may reflect the effect of hormone therapy
(e.g., tamoxifen and aromatase inhibitors) or other
adjuvant treatments.
54
DCIS and LCIS
DCIS is considered a potential precursor to invasive
cancer, and is also associated with an increased risk for
developing a new invasive breast cancer. Women with a
history of DCIS are about 10 times more likely to be
diagnosed with an invasive breast cancer than women
without DCIS.
55
Although LCIS seldom becomes invasive cancer, it is a
strong indicator of increased risk. Women with LCIS are 7
to 12 times more likely to develop invasive cancer in either
breast than women without LCIS.
56
Women with LCIS
have been estimated to have a 2% annual risk of being
diagnosed with invasive breast cancer.
57
Benign breast disease
Doctors often categorize benign breast conditions into 3
general groups reflecting the associated degree of cancer
risk: nonproliferative lesions, proliferative lesions without
atypia (abnormal cells or patterns of cells), and
proliferative lesions with atypia.
• Nonproliferative lesions are not associated with
overgrowth of breast tissue and include fibrosis
and simple cysts (also known as fibrocystic changes)
and mild hyperplasia. Nonproliferative conditions
are associated with little to no increased breast
cancer risk.
58
• Proliferative lesions without atypia are associated
with a small increase in the risk of breast cancer (1.5
to 2 times the risk of those who do not have one of
these lesions) and include usual ductal hyperplasia
(without atypia) and fibroadenoma.
58
• Proliferative lesions with atypia are associated with
about 4 times higher than average risk. These include
atypical ductal hyperplasia and atypical lobular
hyperplasia.
58
Benign breast conditions are most strongly associated
with risk for HR+ breast cancers. Women should keep
detailed records of any benign breast biopsy results,
as they are valuable for risk assessment, screening,
and counseling for chemoprevention and other risk-
reduction strategies.
Table 4. Factors That Increase the Relative Risk for
Breast Cancer in Women
Relative
Risk Factor
>4.0 Age (65+ versus <65 years, although risk increases across
all ages until age 80)
Biopsy-confirmed atypical hyperplasia
Certain inherited genetic mutations for breast cancer
(BRC A1 and/or BRCA2)
Ductal carcinoma in situ
Lobular carcinoma in situ
Mammographically dense breasts (compared to least
dense)
Personal history of early-onset (<40 years) breast cancer
Two or more first-degree relatives with breast cancer
diagnosed at an early age
2.1- 4.0 Personal history of breast cancer (40+ years)
High endogenous estrogen or testosterone levels
(postmenopausal)
High-dose radiation to chest
One first-degree relative with breast cancer
1.1-2.0 Alcohol consumption
Ashkenazi Jewish heritage
Diethylstilbestrol exposure
Early menarche (<12 years)
Height (tall)
High socioeconomic status
Late age at first full-term pregnancy (>30 years)
Late menopause (>55 years)
Never breastfed a child
No full-term pregnancies
Obesity (postmenopausal)/adult weight gain
Personal history of endometrium or ovarian cancer
Proliferative breast disease without atypia (usual ductal
hyperplasia and fibroadenoma)
Recent and long-term use of menopausal hormone therapy
containing estrogen and progestin
Recent oral contraceptive use
14 Breast Cancer Facts & Figures 2017-2018
Breast density
Breast tissue density is a mammographic indicator of the
amount of glandular and connective tissue relative to
fatty tissue. Compared to women with 11%-25% breast
density, those with 26%-50% or 50% or greater breast
density have about a 1.6 or 2.3 times, respectively, higher
risk of breast cancer.
59
About 43% of US women ages 40-74
have heterogeneously dense or extremely dense breasts
(BI-RADS C or D).
60
Breast density is influenced by
genetics, but generally decreases with age, pregnancy,
menopause, and higher body weight.
61, 62
Some drugs also
affect breast density, including tamoxifen (decreases
density) and combined menopausal hormone therapy
(increases density).
63, 64
Mammographic detection of breast cancer is impaired in
areas of dense breast tissue.
65
More than half of US states
now have laws requiring that mammography reports
include information about breast density.
66
Many states
with these laws also require that women with dense
breasts be told that they may benefit from supplemental
imaging tests, such as ultrasound or MRI. Digital breast
tomosynthesis is also useful in evaluating dense breasts.
However, there is currently no expert consensus about
what other tests, if any, should be done in addition to
mammograms to screen for breast cancer in women with
dense breasts.
67, 68
Height
Many studies have found that taller women have a higher
risk of breast cancer than shorter women.
69, 70
A recent study
from Europe found that an increase of 2 inches in height was
associated with about a 10% higher risk of breast cancer
diagnosis and death.
71
Height is also associated with a
number of other cancers, and although the reasons are
not fully understood, it may reflect differences in early
growth as well as hormonal or genetic factors.
Menstrual cycles
Breast cancer risk increases slightly for each year earlier
menstruation begins (by about 5%) and for each year
later menopause begins (by about 3%).
72
For example,
breast cancer risk is about 20% higher among girls who
begin menstruating before age 11 compared to those who
begin at age 13.
72
Likewise, women who experience
menopause at age 55 or older have about a 12% higher
risk compared to those who do so between ages 50-54.
72
The increased risk may be due to longer lifetime exposure
to reproductive hormones and has been more strongly
linked to HR+ breast cancer than other subtypes.
73
Bone mineral density
High bone mineral density in postmenopausal women
has been associated with a 60% to 80% increased risk for
breast cancer compared to low bone density; risk appears
to be most strongly related to HR+ disease.
74, 75
Bone
density is not thought to be an independent risk factor for
breast cancer, but a marker of cumulative estrogen
exposure.
76
However, because bone density is routinely
measured to identify women at increased risk for
osteoporosis (high bone density indicates absence of
osteoporosis), it also may be helpful for identifying
women at increased risk for breast cancer.
Endogenous hormone levels
Postmenopausal women with naturally high levels of
certain endogenous sex hormones have about twice the
risk of developing breast cancer compared to women with
the lowest levels, with the strongest relationships found for
HR+ tumors.
77, 78
High circulating hormone levels are
associated with, and may reflect, the effects of other breast
cancer risk factors, such as postmenopausal obesity and
alcohol use.
78
Although it is challenging to study the
relationship of hormones in premenopausal women
because levels vary across the menstrual cycle, a recent
large review found that high levels of circulating estrogens
and androgens are associated with a small increased risk
of breast cancer in premenopausal women.
79
Reproductive factors
Pregnancy
Having a first child before age 35 and having a greater
number of children is associated with decreased risk of
HR+ breast cancer.
80
In contrast, there appears to be a
transient increase in HR- breast cancer risk (lasting about
10 years) following a full-term pregnancy, particularly
among women who are older at first birth.
81, 82
Breast Cancer Facts & Figures 2017-2018 15
Fertility drugs
More research is needed on the relationship between
breast cancer risk and the long-term effects of ovulation-
stimulating drugs.
83
A long-term follow-up study of
women seen at 5 US fertility clinics found no association
with ever use of clomiphene or gonadotropins; however,
risk of invasive breast cancer was increased among
women who underwent more than 12 clomiphene
treatment cycles compared to women who had never
used fertility drugs.
84
Recently published results of a
long-term follow-up study of Dutch women who used
fertility drugs for in vitro fertilization (IVF), found no
overall association of breast cancer risk with IVF and a
significantly reduced risk of breast cancer among women
who had undergone seven or more IVF cycles.
85
Breastfeeding
Most studies suggest that breastfeeding for a year or more
slightly reduces a woman’s overall risk of breast cancer,
with longer duration associated with greater risk
reduction.
86
In a review of 47 studies in 30 countries, the
risk of breast cancer was reduced by 4% for every 12
months of breastfeeding.
87
One possible explanation for
this effect may be that breastfeeding inhibits
menstruation, thus reducing the lifetime number of
menstrual cycles.
88
Another possible explanation relates
to structural changes that occur in the breast following
lactation and weaning.
86
The protective effect may be
stronger for or even limited to triple negative cancers.
86, 89-90
Hormonal birth control
Studies suggest that recent use of oral contraceptives
(combined estrogen and progesterone) is associated with
a small increase in breast cancer risk, particularly among
women who begin use before 20 years of age or before
first pregnancy.
91
Risk appears to diminish when women
stop use, and after about 10 years, is similar to those who
have never taken oral contraceptives. Most of this
research considered high-dose estrogen formulations,
which were more common in the past. It is unclear if
newer, low-dose estrogen formulations increase breast
cancer risk.
Some, but not all, studies have found recent use of the
injectable progestin-only contraceptive depot-
medroxyprogesterone acetate (Depo-Provera) to be
associated with increased risk of breast cancer; however,
no association has been found with prior use (5 or more
years ago).
92-94
Studies of the levonorgestrel-releasing
intrauterine device (Mirena) have also produced
conflicting results.
95-98
Depo-Provera and Mirena have only
been in use since the 1990s, thus studies with additional
years of follow-up data are needed. Importantly, overall
breast cancer risk is low in young women, and most
studies suggest that any elevation in risk is temporary.
Postmenopausal hormones
Recent use of menopausal hormones (also referred to as
hormone therapy or hormone replacement therapy) with
combined estrogen and progestin increases the risk of
breast cancer, with higher risk associated with longer
use.
99, 100
Risk is also greater for women who start hormone
therapy soon after the onset of menopause compared to
those who begin later.
99, 101
Although discontinuation of
hormone use diminishes breast cancer risk, some increase
in risk seems to persist.
102
The increased risk associated
with estrogen and progestin therapy may be largely due
to increased mammographic density.
65
Postmenopausal estrogen-only therapy has been
associated with uterine problems (including endometrial
cancer), and is therefore only given to women who have
previously undergone hysterectomy. The effects of
estrogen-only therapy on breast cancer risk is less clear.
The US Preventive Services Task Force has concluded that
the use of estrogen alone is associated with reduced risk of
breast cancer based on results from the Women’s Health
Initiative randomized trial, which found that women who
used estrogen-only therapy for an average of 6 years had a
23% lower risk of developing breast cancer.
104
It should be
noted, however, that some observational studies have
found a slight increase in breast cancer risk among
estrogen therapy users, particularly among lean women
and those who begin therapy soon after menopause.
101, 105, 106
Conflicting results may reflect higher rates of screening
in menopausal hormone users, which were not controlled
for in the observational studies.
107

16 Breast Cancer Facts & Figures 2017-2018
Obesity, physical activity, and diet
Obesity and weight gain
Postmenopausal breast cancer risk is about 1.5 times
higher in overweight women and about 2 times higher in
obese women than in lean women.
108
This is likely due, in
part, to higher estrogen levels because fat tissue is the
largest source of estrogen in postmenopausal women, but
may also be related to other mechanisms, including the
higher levels of insulin among obese women.
109, 110
Obesity
is a risk factor for type II diabetes, which has also been
linked to increased risk for postmenopausal breast
cancer.
111, 112
A review of 40 studies concluded that breast
cancer risk was 16% higher in women with type II
diabetes independent of obesity.
113
Weight gain also increases risk of postmenopausal breast
cancer. A large meta-analysis recently concluded that each
5 kg (about 11 pounds) gained during adulthood increases
risk of postmenopausal breast cancer by 11%.
114
Notably,
the increased risk was only observed among women who
did not use menopausal hormones. Although some
studies have found weight loss to be associated with
reduced risk, results are inconsistent.
115-117
It is more
difficult to examine the effect of weight loss because it is
often not sustained.
In contrast, studies have found that obesity protects
against premenopausal breast cancer. A large meta-
analysis found that among women between 40 and 49
years of age, the risk for developing breast cancer was
about 14% lower in overweight women and 26% lower in
obese women compared to women who were normal
weight.
118
The underlying mechanisms for this inverse
relationship are not well understood, but the protective
effect may be limited to HR+/luminal A breast cancers.
43
Physical activity
Women who get regular physical activity have a 10%-20%
lower risk of breast cancer compared to women who are
inactive.
119
The protective effect is independent of BMI
and may be limited to women who have never used
menopausal hormone therapy.
119
A greater reduction in
risk is associated with increasing amounts of exercise and
more vigorous activity; however, even smaller amounts of
exercise, including walking, appear beneficial.
120
An
American Cancer Society study that included more than
73,000 postmenopausal women found that breast cancer
risk was 14% lower among women who reported walking 7
or more hours per week compared to women who walked
3 or less hours per week.
120
The benefit may be due to the
effects of physical activity on systemic inflammation,
hormones, and energy balance.
119, 121
What is the difference between absolute,
lifetime, and relative risks?
Absolute risk: Absolute risk is the likelihood of being
diagnosed with cancer over a certain period of time.
For example, 22 out of 10,000 women ages 50-54 will
be diagnosed with breast cancer in the next year.
Lifetime risk: Lifetime risk is the absolute risk of being
diagnosed with cancer over the course of a lifetime
from birth to death. Lifetime risk of breast cancer
reflects the average probability of a female being
diagnosed with breast cancer in the US. A woman
living in the US has a 12% chance of being diagnosed
with breast cancer in her lifetime (Table 2, page 4).
Another way to say this is that 1 out of every 8 women
will be diagnosed with breast cancer in her lifetime.
Relative risk: Relative risk compares the absolute risk
of disease among people with a particular risk factor
to the risk among people without that risk factor. If
the relative risk is above 1.0, then risk is higher among
those with the risk factor than among those without
the factor. Relative risks below 1.0 reflect an inverse
association between the exposure and the disease,
or a protective effect. For example, one study found
women ages 50-59 who were current users of combined
estrogen and progestin menopausal hormones had
a relative risk of developing breast cancer of 1.21, or
a 21% increased risk compared to women who have
not used hormone therapy.
100
While relative risks are
useful for comparisons, they do not provide information
about the absolute risk of the exposed group. In this
example, 33 breast cancers per year would be expected
to be diagnosed among 10,000 women ages 50-59
who use estrogen and progestin (that is the absolute
risk among this group). Among 10,000 women of the
same ages who never used menopausal hormones, 27
cases per year would be expected. Therefore, the 21%
increased relative risk results in a total of 6 additional
breast cancers diagnosed per 10,000 women per year.
Breast Cancer Facts & Figures 2017-2018 17
Diet
Numerous studies have examined the relationship
between food consumption (including fat, fiber, soy,
dairy, meat, and fruits and vegetables) and breast cancer
with mixed results. Although early diet and breast cancer
studies focused on fat intake, a recent meta-analysis
concluded there was no association.
122
It has been
suggested that soy consumption may reduce breast cancer
risk, in part because of historically low breast cancer rates
among Asian women. A meta-analysis showed that soy
intake was inversely associated with breast cancer risk in
Asian but not Western populations, perhaps because
Asian women generally consume more soy products
beginning at an earlier age than Western women.
123
There is growing evidence that high levels of fruit and/or
vegetable consumption may reduce the risk of HR- breast
cancer.
124-126
These findings are supported by studies
linking lower breast cancer risk to higher blood levels
of carotenoids (micronutrients found in fruit and
vegetables).
127-129
The effect of diet on breast cancer risk
remains an active area of research, with studies
particularly focused on timing of exposure, specific
dietary components, and risk differences by tumor
hormone receptor status.
Alcohol
Numerous studies have confirmed that alcohol
consumption increases the risk of breast cancer in
women by about 7%-10% for each 10g (roughly one drink)
of alcohol consumed per day on average.
41
Women who
have 2-3 alcoholic drinks per day have a 20% higher risk
of breast cancer compared to non-drinkers. There is also
evidence that alcohol consumption before first pregnancy
may particularly affect risk.
41, 130
One of the mechanisms
by which alcohol increases risk is by increasing estrogen
and androgen levels.
131
Alcohol use appears more strongly
associated with increased risk for HR+ than HR- breast
cancers.
132
Tobacco
Accumulating research indicates that smoking may
slightly increase breast cancer risk, particularly long-
term, heavy smoking and among women who start
smoking before their first pregnancy.
133-136
The 2014 US
Surgeon General’s report on smoking concluded that
there is “suggestive but not sufficient” evidence that
smoking increases the risk of breast cancer.
137
A review by
American Cancer Society researchers found that women
who initiated smoking before the birth of their first child
had a 21% higher risk of breast cancer than women who
never smoked.
135
Some studies suggest secondhand
smoke may increase risk, particularly for premenopausal
breast cancer.
133, 134
Environmental and other risk factors
Radiation
Radiation exposure has been shown to increase breast
cancer risk in studies of atomic bomb survivors and
females treated with high-dose radiation therapy to the
chest between 10 and 30 years of age, such as for Hodgkin
lymphoma.
138, 139
This may be because breast tissue is
most susceptible to carcinogens before it is fully
differentiated, which occurs with first childbirth.
140
Breast cancer risk starts to rise about 8 years after
radiation treatment and continues to be elevated for
more than 35 years.
139, 141
Although radiation treatments
have evolved to include lower doses given over smaller
areas, recent studies suggest that the elevated breast
cancer risk persists.
141, 142
Diethylstilbestrol exposure
From the 1940s through the 1960s, some pregnant
women were given the drug diethylstilbestrol (DES)
because it was thought to lower the risk of miscarriage.
These women have an increased risk (about 30%) of
developing breast cancer compared to women who have
not taken DES.
143
Some studies also suggest that women
whose mothers took DES during pregnancy also have a
slightly higher risk of breast cancer.
144
Environmental pollutants
In general, epidemiological studies have not found clear
relationships between environmental pollutants, such as
organochlorine pesticides, and breast cancer. Studies to
date have found no association between increased
concentrations of organochlorines (e.g., dichlorodiphenyl-
18 Breast Cancer Facts & Figures 2017-2018
trichloroethane or DDT) in blood and fat tissue and
breast cancer risk,
145-148
although a recent study found in
utero exposure to DDT was linked to breast cancer risk
later in life.
149
Animal studies have demonstrated that
prolonged, high-dose exposure to many industrial
chemicals can increase mammary tumor development,
but it is unknown whether the much lower dose
exposures that occur in the general environment in air,
drinking water, and consumer products increase human
breast cancer risk.
150
Night shift work
Most studies of nurses who work night shifts and flight
attendants who experience circadian rhythm disruption
caused by crossing multiple time zones have found
increased risks of breast cancer associated with long-
term employment.
151, 152
Elevated risk appears to be most
strongly associated with shift working during early
adulthood.
153
Exposure to light at night disrupts the
production of melatonin, a hormone that regulates sleep.
Experimental evidence suggests that melatonin may also
inhibit the growth of small, established tumors and
prevent new tumors from developing.
154
Based on the
results of studies in humans and animals, the
International Agency for Research on Cancer concluded
in 2007 that shift work, particularly at night, was
probably carcinogenic to humans.
155
Shift work at night is
a common exposure, involving about 15% to 20% of
workers in the US and Europe, and much of the
population in industrialized countries is exposed to
artificial light at night.
Factors that are not associated
with breast cancer risk
Abortion
There are persistent claims that women who have had an
abortion are at increased risk for developing breast cancer
based on early studies that have since been deemed
methodologically flawed by the American College of
Obstetricians and Gynecology.
156
Indeed, a large body of
solid scientific evidence, including a review by a panel of
experts convened by the National Cancer Institute in
2003, confirms that there is no link between breast
cancer and abortion (either spontaneous or induced).
157
Bras
Although internet rumors have suggested that bras cause
breast cancer by obstructing lymph flow, there is no
scientific basis or evidence to support this claim. A recent
population-based study of more than 1,500 women found
no association between wearing a bra and breast cancer.
158
Breast implants
No association has been found between breast implants
and risk of breast cancer; however, there is evidence that
women with implants are at increased risk of a rare type of
lymphoma.
159
Breast implants can also obstruct the view of
breast tissue during mammography. A woman with breast
implants should inform the mammography facility about
the implants during scheduling so that additional x-ray
pictures (called implant displacement views) may be used
to allow for more complete breast imaging.
Hair dyes, relaxers, and antiperspirants
Although one recent study suggested that selected hair
products may be associated with breast cancer, most
studies have failed to reveal any correlation.
160
A
combined analysis of 14 studies found no association
between the use of permanent hair dyes and breast
cancer.
161
A study of more than 48,000 black women found
no link to breast cancer with use of hair relaxers.
162
Although antiperspirant use has been less well-studied,
there is presently no convincing scientific evidence of an
association with breast cancer.
163, 164
Chemoprevention and
prophylactic surgery
Chemoprevention
The use of drugs to reduce the risk of disease is called
chemoprevention. Currently, the US Food and Drug
Administration (FDA) has approved two drugs for the
prevention of breast cancer in high-risk women:
tamoxifen and raloxifene (postmenopausal women only).
These drugs are classified as selective estrogen receptor
modulators (or SERMs) because they block estrogen in
some tissues of the body, but act like estrogen in others.
A recent meta-analysis, including more than 83,000
high-risk women, found that SERMs reduced breast

Breast Cancer Facts & Figures 2017-2018 19
cancer risk by 38% over 10 years.
165
Although the benefit
is limited to ER+ disease, these drugs lower the risk of
both invasive cancer and ductal carcinoma in situ.
However, SERMs are associated with some side effects,
including hot flashes, nausea, and fatigue. Premenopausal
women taking tamoxifen can also experience menstrual
changes. More serious side effects are rare, but include
blood clots and endometrial cancer.
165
Clinical trials are examining another class of drugs –
aromatase inhibitors – to see if they may also be effective
for reducing breast cancer risk among postmenopausal
women. Currently, these drugs are only approved to
prevent breast cancer recurrence. Aromatase inhibitors
target the enzyme responsible for producing estrogen in
fat tissue, and thus are only effective in women without
functioning ovaries (e.g., postmenopausal women),
because ovaries are the primary source of estrogen before
menopause. Early clinical trial results are promising:
breast cancer risk was reduced by more than half in
high-risk women taking anastrozole or exemestane
compared to placebo.
166, 167
Women taking aromatase
inhibitors must be monitored for osteoporosis, as these
medications can decrease bone density.
Prophylactic surgery
Women at very high risk of breast cancer (such as those
with BRCA gene mutations) may elect prophylactic
(preventive) mastectomy. This operation removes one or
both breasts. Removing both breasts before cancer is
diagnosed reduces the risk of breast cancer by 90% or
more.
168
Prophylactic salpingo-oophorectomy (surgical
removal of the fallopian tubes and ovaries) has also been
shown to reduce the risk of both breast and ovarian
cancers,
169, 170
but a recent study found that the breast
cancer benefit may be limited to women who carry
BRCA2 mutations.
171
Importantly, however, not all women
who elect to have these surgeries would have developed
cancer. A woman considering prophylactic surgery
should discuss the benefits and limitations with her
doctor and a second opinion is strongly recommended.
See page 25 for further discussion of contralateral
prophylactic mastectomy.
Breast Cancer Screening
American Cancer Society recommendations for the early
detection of breast cancer vary depending on a woman’s
age and include mammography, as well as magnetic
resonance imaging (MRI) for women at high risk. In 2015,
the American Cancer Society updated its breast cancer
screening guideline for average-risk women.
172
Mammography
Mammography is a low-dose x-ray procedure that allows
visualization of the internal structure of the breast. There
are three main types of mammography: screen-film,
digital, and digital breast tomosynthesis. Screen-film
mammography uses x-ray equipment to record images.
Digital mammography, which uses more specialized
computerized equipment to capture a digital image of the
breast and delivers lower doses of radiation, has largely
replaced film mammography. Studies have shown that
digital mammograms are more accurate for women under
the age of 50 and those with dense breast tissue.
173-175
In 2011, the FDA approved the use of digital breast
tomosynthesis or 3-dimensional (3-D) mammography,
which constructs a 3-D image of the breast with multiple
high-resolution x-rays, to be used in combination with a
2-D digital mammography image. The benefits and
limitations of tomosynthesis in community practice are
still being assessed. Recent studies suggest that the
addition of breast tomosynthesis to digital mammography
may reduce false positives and slightly improve cancer
detection compared to digital mammography alone.
176-178
However, when the 2-D images are produced separately
from the tomographic images, women receive about twice
the radiation dose. Recently, the FDA approved the use of
tomographic images to produce synthetic, conventional
2-D images, thus reducing the radiation dose to that
similar to conventional digital mammography. This

20 Breast Cancer Facts & Figures 2017-2018
newer type of mammographic screening is not yet
available in all communities and may not be fully covered
by health insurance.
For women at average-risk of breast cancer, the American
Cancer Society recommends that those 40 to 44 years of
age have the option to begin annual mammography; those
45 to 54 years should undergo annual mammography;
and those 55 years of age or older may transition to
biennial mammography or continue with annual
mammograms. Women should continue screening as
long as their overall health is good and they have a life
expectancy of 10 years or more.
It is especially important that women are regularly
screened to increase the chance that a breast cancer is
detected early before it has spread. Recommended
screening intervals are based on the duration of time a
breast cancer is detectable before symptoms develop.
Combined results from randomized controlled screening
trials suggest that mammography reduces the risk of
dying from breast cancer by about 20%, whereas studies
of modern mammography screening programs in Europe
and Canada found that the risk of breast cancer death
among women exposed to screening was reduced by
more than 40%.
179-181
Early detection of breast cancer by
mammography also leads to a greater range of treatment
options, including less-extensive surgery (e.g., breast-
conserving surgery like lumpectomy versus mastectomy)
and the use of chemotherapy with fewer serious side effects,
or sometimes, the option to forgo chemotherapy.
However, mammography screening does have limitations
or potential harms, which are described below.
The Affordable Care Act requires that Medicare and all
new health insurance plans fully cover screening
mammograms without any out-of-pocket expense for
patients. For help locating a free or low-cost screening
mammogram in your area, contact the American Cancer
Society at 1-800-227-2345.
False-positive results
Mammography sometimes leads to follow-up
examinations, including biopsies, when there is no
cancer, referred to as false-positive test results. A false
positive is most likely following a woman’s initial
screening mammogram.
182
Other factors that increase
the likelihood of a false positive include the use of
postmenopausal hormone therapy and having more
American Cancer Society Guideline for
Breast Cancer Screening, 2015
172
These recommendations represent guidance
from the American Cancer Society for women at
average risk of breast cancer, i.e., women without
a personal history of breast cancer, a suspected
or confirmed genetic mutation known to increase
risk of breast cancer (e.g., BRCA), or a history of
previous radiotherapy to the chest at a young age.
We recommend that all women should become
familiar with the potential benefits, limitations, and
harms associated with breast cancer screening.
Recommendations*:
1. Women with an average risk of breast cancer
should undergo regular screening mammography
starting at age 45 years (strong recommendation).
• Women should have the opportunity to
begin annual screening between the ages of
40 and 44 (qualified recommendation).
• Women who are age 45 to 54 should be screened
annually (qualified recommendation).
• Women who are age 55 and older should transition to
biennial screening or have the opportunity to continue
screening annually (qualified recommendation).
2. Women should continue screening
mammography as long as their overall health
is good and they have a life expectancy of 10
years or more (qualified recommendation).
3. The American Cancer Society does not
recommend clinical breast examination for breast
cancer screening among average-risk women
at any age (qualified recommendation).
*A strong recommendation conveys the consensus that the benefits of
adherence to that intervention outweigh the undesirable effects that may
result from screening. Qualified recommendations indicate there is clear
evidence of the benefit of screening but less certainty about either the balance
of benefits and harms, or about patients’ values and preferences, which
could lead to different decisions about screening.

Breast Cancer Facts & Figures 2017-2018 21
mammographically dense breast tissue.
182, 183
On average,
1 in 9 women are recalled from each screening
examination for further testing (most often additional
mammographic views), but most (95%) do not have
cancer.
184
According to one US study, over the course
of 10 screening examinations, about one-half of women
experience a false positive, and about 19% undergo biopsy
but do not have cancer.
185
Overdiagnosis
Mammography likely results in some overdiagnosis; that
is, the diagnosis of cancer that would not cause a woman
any harm in her lifetime and that would not have
progressed or otherwise been detected in the absence of
screening. There are two circumstances that can lead to
overdiagnosis. The first is a breast cancer is diagnosed by
screening in a woman who dies shortly afterward from a
cause other than breast cancer. National guidelines
recommend against screening in women who are very ill
or have limited life expectancy. The second, which is
more difficult to measure, is the detection of a truly
non-progressive in situ or invasive cancer. Estimates of
the prevalence of overdiagnosis are highly variable,
ranging from <5% to more than 30%.
186-191
Radiation exposure
The dose of radiation during a mammogram is very small
and the risk of harm is minimal.
192, 193
Limitations of mammography
Not all breast cancer will be detected early by a
mammogram, and some cancers that are screen-detected
still have poor prognosis. Most women will never be
diagnosed with breast cancer, but will undergo regular
screening and may experience one or more “false alarms.”
In an effort to maximize the benefits and minimize the
harms of screening, some scientists are attempting to
determine which factors could be used to individualize
screening recommendations (e.g., which women could
start screening at older ages and/or be screened less
often).
194
Despite these limitations, mammography is the single
most effective method of early breast cancer detection
since it can often identify cancer several years before
physical symptoms develop. It is the position of the
American Cancer Society that the balance of benefits to
possible harms strongly supports the value of regular
breast cancer screening in women for whom it is
recommended.
Table 5. Prevalence of Mammography (%), Women
40 and Older, US, 2015
Characteristic
Within
the past
year
Within
the past
two years
Overall 50 64
Age (years)
40-44 38 49
45-54 54 69
55+ 53 68
Race/Ethnicity
Non-Hispanic White 50 65
Non-Hispanic Black 55 69
Asian American 47 59
American Indian and Alaska Native 46 60
Hispanic/Latina 46 61
Education
Some high school or less 39 51
High school diploma or GED 45 58
Some college/Assoc. degree 51 66
College graduate 58 73
Sexual orientation
Gay/Lesbian 62 78
Straight 50 64
Bisexual * *
Health insurance status (ages 40-64)
Uninsured 21 31
Insured 53 68
Immigration
Born in US 51 66
Born in US territory 47 59
In US fewer than 10 years 33 46
In US 10 or more years 47 60
Region
Northeast 54 67
Midwest 51 63
South 50 65
West 47 63
GED = General Educational Development high school equivalency. *Estimate
not provided due to instability. Note: Estimates are age adjusted to the 2000 US
standard population. Mammography prevalence estimates do not distinguish
between examinations for screening and diagnosis.
Source: National Health Interview Survey, 2015.
American Cancer Society, Surveillance Research, 2017
22 Breast Cancer Facts & Figures 2017-2018
Prevalence of mammography
According to the 2015 National Health Interview Survey,
50% of women 40 years of age and older reported having
had a mammogram within the past year and 64%
reported having a mammogram in the past 2 years
(Table 5, page 21).
195
Among women 40 years of age and
older, mammography prevalence increased from 29% in
1987 to 70% in 2000, and has since gradually declined.
Women who have less than a high school education, who
have no health insurance coverage, or who are recent
immigrants to the US are least likely to have had a recent
mammogram. Efforts to increase screening should
specifically target socioeconomically disadvantaged
women and recent immigrants.
Table 6 shows the percentage of US women 40 years of
age and older who have had a recent mammogram by
state, based on data from the 2014 Behavioral Risk Factor
Surveillance System.
196
Among women of all races
combined 40 years of age and older, reported rates of
mammograms in the past 2 years range from 62% in
Idaho to 82% in Massachusetts.
The Centers for Disease Control and Prevention’s
National Breast and Cervical Cancer Early Detection
Program (NBCCEDP) was established in 1990 to improve
access to breast cancer screening and diagnostic services
for low-income women and was recently shown to help
save lives from breast cancer.
197
However, the CDC
estimates that the program is currently only reaching
about 11% of eligible women due in part to funding
shortages.
198
Magnetic resonance imaging (MRI)
An expert panel convened by the American Cancer
Society published recommendations for the use of MRI
for screening women at increased risk for breast cancer in
2007.
199
The panel recommended annual MRI screening
in addition to mammography for women at high lifetime
risk (~20%-25% or greater) beginning at 30 years of age.
Women at moderately increased risk (15%-20% lifetime
risk) should talk with their doctors about the benefits
and limitations of adding MRI screening to their yearly
mammogram. MRI screening is not recommended for
women whose lifetime risk of breast cancer is less than
15%. Studies indicates that although MRI is underutilized
among high-risk women, it is often used in women who
are not at high risk for breast cancer.
200
MRI uses magnetic fields instead of x-rays to produce
very detailed, cross-sectional images of the body. A
contrast material (usually gadolinium) is injected into a
vein to improve the ability to capture detailed images of
breast tissue. It is important that screening MRIs are
done at facilities that can perform an MRI-guided breast
biopsy if abnormalities are found. Otherwise, the scan
must be repeated at another facility if a biopsy is
necessary. Although MRI is more expensive than
mammography, most major insurance companies will
cover some portion of the costs if a woman can be shown
to be at high risk. MRIs should supplement, but not
replace, mammography screening.
Breast ultrasound
Breast ultrasound is sometimes used to evaluate abnormal
findings from a mammogram or physical exam. Studies
have shown that ultrasound detects more cancer than
mammography alone when screening women with
mammographically dense breast tissue; however, it also
increases the likelihood of false-positive results.
67, 201
The
use of ultrasound instead of mammograms for breast
cancer screening is not recommended.
Clinical breast examination (CBE)
The American Cancer Society no longer recommends
CBE for average-risk asymptomatic women based on lack
of clear benefits for CBE alone or in conjunction with
mammography. Compared to mammography alone, CBE
plus mammography has been shown to detect only a
small additional proportion of breast cancer tumors and
increases the probability of false positives.
202, 203
Breast self-awareness
Although the American Cancer Society no longer
recommends that all women perform monthly breast
self-exams (BSE), all women should become familiar with
both the appearance and feel of their breasts and report

Breast Cancer Facts & Figures 2017-2018 23
Table 6. Prevalence of Mammography* (%) by State, Women 40 and Older, 2014
Within the past year Within the past two years
All NH White NH Black
Uninsured
(Ages
40-64) All NH White NH Black
Uninsured
(Ages
40-64)
United States (median)* 56 56 60 27 73 72 77 43
Range 45-68 45-68 40-71 16-46 62-82 62-83 55-89 29-68
Alabama 57 55 65 27 73 71 80 39
Alaska 45 45 * 25 63 62 * 40
Arizona 54 54 55 38 71 72 74 51
Arkansas 49 49 53 21 65 65 66 29
California 60 59 71 44 77 77 89 56
Colorado 51 52 58 28 69 69 86 44
Connecticut 64 64 65 34 80 80 82 54
Delaware 63 64 63 37 79 80 78 68
District of Columbia 53 52 56 * 75 72 79 *
Florida 58 58 62 27 74 74 79 46
Georgia 60 57 67 35 75 73 81 52
Hawaii 65 62 * 34 79 76 * 52
Idaho 47 47 * 19 62 63 * 34
Illinois 55 55 56 17 74 72 79 46
Indiana 52 52 58 22 67 67 74 35
Iowa 62 62 * 28 76 76 * 37
Kansas 56 57 57 26 71 72 75 39
Kentucky 61 60 64 27 75 74 78 37
Louisiana 58 56 61 36 75 74 77 53
Maine 63 63 * 28 78 79 * 43
Maryland 63 62 70 41 79 78 84 60
Massachusetts 68 68 64 46 82 83 75 59
Michigan 58 58 59 26 76 76 80 44
Minnesota 61 61 63 39 77 77 75 56
Mississippi 53 53 58 29 68 67 71 42
Missouri 55 54 64 25 68 67 77 34
Montana 50 51 * 23 69 69 * 41
Nebraska 53 54 56 16 70 71 69 30
Nevada 52 51 40 22 70 69 54 44
New Hampshire 62 62 * 35 79 79 * 51
New Jersey 59 57 64 33 74 74 75 51
New Mexico 49 50 * 24 66 67 * 38
New York 60 59 63 40 75 74 78 53
North Carolina 63 63 65 28 77 77 78 46
North Dakota 56 57 * 33 72 73 * 43
Ohio 56 55 65 25 72 71 82 35
Oklahoma 51 52 51 23 66 66 67 37
Oregon 54 54 * 26 70 71 * 36
Pennsylvania 57 57 60 19 73 73 77 37
Rhode Island 65 66 54 34 81 81 78 47
South Carolina 54 53 59 22 72 71 77 36
South Dakota 61 61 * 27 75 75 * 48
Tennessee 56 55 61 22 73 72 78 37
Texas 54 55 58 35 71 71 76 51
Utah 49 50 * 19 66 67 * 33
Vermont 56 57 * 25 74 75 * 37
Virginia 60 58 69 31 75 74 84 51
Washington 53 54 54 20 71 72 72 32
West Virginia 56 56 50 20 72 72 71 31
Wisconsin 59 60 57 * 74 75 67 32
Wyoming 47 47 * 21 65 66 * 40
NH: non-Hispanic. *Estimate not provided due to instability.
Source: Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System, public use data file, 2014.
©2017 American Cancer Society, Inc., Surveillance Research

24 Breast Cancer Facts & Figures 2017-2018
any changes promptly to their physician. Experts have
concluded that self-awareness seems to be at least as
effective for detecting breast cancer as structured BSE.
204-206
If symptoms develop, women should contact a doctor
immediately, even after a recent normal mammogram.
However, most lumps are not abnormal, and for women
who are still menstruating, they can appear and
disappear with the menstrual cycle. Most breast lumps
are not cancerous.
Breast Cancer Treatment
Treatment decisions are made jointly by the patient and
the physician after consideration of the stage and
biological characteristics of the cancer, the patient’s age,
menopausal status, and preferences, and the risks and
benefits associated with each option.
In Situ
Since there is no certain way to determine the progressive
potential of a DCIS lesion, surgery and sometimes
radiation and/or hormone therapy is the usual course of
action following a diagnosis of DCIS. However, there may
be a group of patients that could safely forgo surgical
treatment for DCIS. Several clinical trials are underway
that are comparing standard treatment to active
monitoring in women with low-risk DCIS.
207-209
Research
is also ongoing to identify molecular markers of DCIS
that could predict recurrence or progression to invasive
cancer.
210
Classic LCIS does not require surgical treatment, but
there is no consensus about optimal treatment for more
aggressive (pleomorphic) LCIS.
56, 211
Invasive
Figure 11 shows treatment patterns among US women
with invasive breast cancer in 2013. Most women with
early-stage breast cancer will have some type of surgery,
which is often combined with other treatments to reduce
the risk of recurrence, such as radiation therapy,
chemotherapy, hormone therapy, and/or targeted
therapy. Patients with metastatic disease are primarily
treated with systemic therapies, which can include
chemotherapy, targeted therapy, and hormonal therapy.
Surgery
The primary goals of breast cancer surgery are to remove
the cancer and determine its stage. Surgical treatment
involves breast-conserving surgery (BCS) or mastectomy.
With BCS (also known as partial mastectomy or
lumpectomy), only cancerous tissue, plus a rim of normal
tissue (tumor margin), is removed. BCS is generally not
an option in those with a high tumor-to-breast ratio,
those with multicentric cancers, or those with
inflammatory or locally advanced cancers. In most cases,
BCS needs to be followed by radiation to the breast, and
thus patients who are not candidates for breast
radiotherapy, such as those who had previous breast
radiation, are also not candidates for BCS. Simple or total
mastectomy includes removal of the entire breast.
Modified radical mastectomy includes removal of the
entire breast, plus a full axillary lymph node dissection
(see below for discussion of lymph node procedures).
Radical mastectomy is rarely performed anymore
because removal of the underlying chest muscles is not
necessary to remove all of the cancer in most patients.
Long-term results of multiple international, randomized
clinical trials have found equivalent survival for the
majority of patients with stage I or II breast cancer who
have BCS followed by radiation or mastectomy.
212, 213
Some more recent, observational studies even suggest
possible improved survival and reduced recurrence rates
with BCS.
214-217
In addition, risk of complications is nearly
twice as high for women who undergo mastectomy with
reconstruction compared to BCS plus radiation.
218
Nevertheless, many BCS-eligible women continue to
undergo mastectomy.
219, 220
Reasons include reluctance to
undergo radiation therapy after BCS and fear of
recurrence.
221
Younger women (those under 40 years of

Breast Cancer Facts & Figures 2017-2018 25
age), patients with larger and/or more aggressive tumors,
and those who live farther from their treatment facility
are also more likely to undergo mastectomy.
219, 221-223
Some women who are diagnosed with breast cancer in
one breast choose to have the unaffected breast removed
as well. This is known as contralateral prophylactic
mastectomy (CPM) or bilateral mastectomy. Recent
studies have shown marked increases in the rate of CPM
for women diagnosed with invasive breast cancer, as well
as DCIS.
220, 224-226
Although CPM greatly reduces the risk of
developing a new breast cancer, it does not improve
long-term breast cancer survival for the vast majority of
women and doubles the risk of surgical complications.
227, 228
Both BCS and mastectomy are usually accompanied by
removal of one or more regional lymph nodes from the
axilla to determine if the disease has spread beyond the
breast and help stage the cancer. The presence of cancer
cells in the lymph nodes increases the risk of recurrence,
and so can help determine the need for further
treatment. Sentinel lymph node biopsy (SLNB) involves
removing and testing selected lymph nodes before any
others are excised. Although cancer in sentinel lymph
nodes was traditionally an indication for additional
axillary lymph node surgery, studies have shown that it
may not be necessary when cancer cells are found only in
1 or 2 sentinel lymph nodes in patients undergoing BCS
with whole breast radiation.
229, 230
A full axillary lymph
node dissection (ALND) is often indicated for patients
with one or more axillary lymph nodes found to contain
cancer prior to surgery. Ongoing clinical trials are
investigating the safety of avoiding ALND in patients who
were initially diagnosed with lymph node-positive breast
cancer, but have small tumors or in whom neoadjuvant
chemotherapy appears to have eliminated cancer in the
lymph nodes. Patients should talk with their doctors to
determine what lymph node procedure is planned for
their surgery.
Surgery (and/or radiation therapy) involving the axillary
lymph nodes can lead to lymphedema, a serious swelling
of the arm caused by retention of lymph fluid. It affects
about 20% of women who undergo ALND and 6% of
patients who receive SLNB.
231
There are several effective
therapies for lymphedema, and some evidence suggests
that upper-body exercise and physical therapy may reduce
the risk and lessen the severity of this condition.
232, 233
Women who undergo mastectomy may have breast
reconstruction, either with a saline or silicone implant,
tissue from another part of the body, or a combination of
the two. Breast reconstruction among US women
undergoing mastectomy has increased from 12% in 1998
Figure 11. Female Breast Cancer Treatment Patterns (%), by Stage, 2013, US
BCS = breast-conserving surgery; RT = radiation therapy; chemo = chemotherapy and includes targeted therapy and immunotherapy drugs.
Source: National Cancer Data Base, 2013.
American Cancer Society, Inc., Surveillance Research, 2017
0
10
20
30
40
50
Stage IVStage IIIStages I and II
Percent
8
1717
1 1 1 1
6
7
13
3 3
48
7 7
21
34
4 4
48
12
15
2 2 2 2 2 2 2 2
BCS alone
BCS + RT
BCS + RT + chemo
BCS + chemo
Mastectomy alone
Mastectomy + RT
Mastectomy + chemo
Mastectomy + RT + chemo
RT and/or chemo
No surgery, RT, or chemo
26 Breast Cancer Facts & Figures 2017-2018
to 36% in 2011.
220
A woman considering breast
reconstruction should discuss this option with her breast
surgeon prior to the mastectomy in order to coordinate
the treatment plan with a plastic surgeon. Some types of
reconstruction can begin during the mastectomy itself,
and reconstruction influences the time spent in the
hospital after a procedure, as well as the recovery time.
The cosmetic appearance of immediate reconstruction
can be negatively affected by subsequent radiotherapy.
Women who do not choose reconstruction prior to
surgery can opt to undergo reconstruction later. Since
1998, the Women’s Health and Cancer Rights Act has
required group health plans, insurance companies, and
health maintenance organizations that offer mastectomy
coverage to also pay for reconstructive surgery.
Reconstruction is also covered by Medicare and
Medicaid, though Medicaid benefits vary by state.
Radiation therapy
Radiation therapy is the use of high-energy beams or
particles to kill cancer cells and is often used after surgery
to destroy cancer cells remaining in the breast, chest
wall, or underarm area. BCS is almost always followed by
radiation therapy to the breast because it has been shown
to reduce the risk of cancer recurrence by about 50% at 10
years and the risk of breast cancer death by almost 20% at
15 years.
213
However, radiation is not necessary in women
70 years of age and older with small, lymph node-negative,
HR+ cancers, because it hasn’t been shown to improve
survival in patients who take hormonal therapy for at
least five years.
234
Some mastectomy-treated patients also
benefit from radiation if their tumor is larger than 5 cm,
growing in to nearby tissues, or if cancer is found in the
lymph nodes. Radiation can also be used to treat the
symptoms of advanced breast cancer, especially when it
has spread to the central nervous system or bones.
Radiation therapy may be administered as external beam
radiation, brachytherapy, or a combination of both. The
method depends on the type, stage, and location of the
tumor, as well as patient characteristics, and doctor and
patient preference. External beam radiation is the
standard type of radiation, whereby radiation from a
machine outside the body is focused on the area affected
by cancer. Traditionally, external beam radiation therapy
is administered 5 days per week over 5 to 7 weeks, but in
select patients a 3-week course appears to be as
effective.
235, 236
Brachytherapy uses a radioactive source
placed in catheters or other devices that are put into the
cavity left after BCS and is sometimes an option for
patients with early-stage breast cancers. Intracavitary
brachytherapy is typically given daily for 5 days.
Accumulating evidence suggests intracavity brachytherapy
may be as effective as whole breast radiation for selected
patients, but deliverable in less time and with fewer side
effects.
237-240
However, most studies of intracavitary
brachytherapy have not followed patients for more than 5
years, so its long-term efficacy as compared to whole
breast radiation has not been established.
For intraoperative radiation therapy, a single dose of
radiation is administered to the tumor bed during
breast-conserving surgery. It can be used instead of
intracavitary brachytherapy but is only available at
limited centers.
241
Systemic therapy
Systemic therapy is treatment that travels through the
bloodstream and affects and treats almost all parts of the
body. Systemic therapy includes chemotherapy, hormone
therapy, and targeted therapy, all of which work through
different mechanisms. For example, chemotherapy drugs
generally work by attacking cells that grow quickly, such
as cancer cells. Hormone therapy works by either blocking
the body’s natural hormones or lowering the levels of
those hormones, which can act to promote the growth of
some cancers. Targeted drugs work by attacking specific
molecules in or on cells that may be more common or
active in cancer cells.
When systemic treatment is given to patients before
surgery, it is called neoadjuvant or preoperative therapy.
For larger breast tumors, it is often used to shrink the
tumor enough to make surgical removal easier and less
extensive (such as BCS in women who would otherwise
have required mastectomy). Neoadjuvant systemic
therapy has been found to be as effective as the same
therapy given after surgery in terms of survival and distant
recurrence.
242
Systemic treatment given to patients after
surgery is called adjuvant therapy. It is used to kill any
Breast Cancer Facts & Figures 2017-2018 27
undetected tumor cells (micrometastases) that may have
migrated to other parts of the body. Systemic therapy is
the main treatment option for women with metastatic
breast cancer.
Chemotherapy
The benefit of chemotherapy is dependent on multiple
factors, including the size of the tumor, the number of
lymph nodes involved, the presence of estrogen or
progesterone receptors, and the presence of HER2
overexpression on the cancer cells. Triple negative and
HER2+ breast cancers tend to be more sensitive to
chemotherapy than HR+ tumors.
243
There are also gene
expression panels (such as Oncotype DX, PAM 50, and
MammaPrint) that can help assess the risk of distant
recurrence in women with early-stage, HR+, HER2- breast
cancers, and potentially identify those who would more
likely benefit from chemotherapy, as well as those who
could safely avoid it. The Oncotype Dx 21-Gene
Recurrence Score is used most widely in the United
States; a high score identifies women who would more
likely benefit from adjuvant chemotherapy (in addition to
hormone therapy) whereas a low score identifies women
who could safely avoid it.
244
These scores are independent
of patient age and tumor size. Clinical trials are currently
underway to further evaluate the predictive value of
some of these tests in women with intermediate risk
scores and those with node positive disease.
Research has established that combinations of drugs are
more effective than one drug alone for treatment of
early-stage breast cancer, and several options exist when
selecting a chemotherapy regimen. Depending on the
combination of drugs used, adjuvant and neoadjuvant
chemotherapy is usually given for 3 to 6 months. This
treatment is most effective when the full dose and cycle
of drugs are completed in a timely manner, without
significant delays or interruption.
Hormone (anti-estrogen) therapy
Estrogen, a hormone produced by the ovaries in addition
to other tissues, promotes the growth of HR+ breast
cancers. Patients with these tumors can be given
hormone therapy to lower estrogen levels or block the
effects of estrogen on the growth of breast cancer cells.
These drugs are different than menopausal hormone
therapies, which actually increase hormone levels.
Hormone therapy for breast cancer can be different in
premenopausal and postmenopausal women.
Tamoxifen is a treatment that blocks the effects of
estrogen in breast tissue but has estrogenic effects in
other tissues, such as the liver, uterus, and bones.
Tamoxifen can be used to treat both early- and
advanced-HR+ breast cancer in both pre- and
postmenopausal women. Adjuvant treatment of early-
stage HR+ breast cancer with tamoxifen for at least 5
years has been shown to reduce the rate of recurrence by
approximately 40%-50% throughout the first decade, and
reduces breast cancer mortality by about one-third
throughout the first 15 years.
245
More recently, studies
have shown that extended use of adjuvant tamoxifen (10
years versus 5 years) further reduces the risk of breast
cancer recurrence and mortality, so clinical practice
guidelines now recommend consideration of adjuvant
tamoxifen therapy for 10 years.
247
Aromatase inhibitors (AIs), such as letrozole, anastrozole,
and exemestane, are another class of drugs used to treat
both early- and advanced-HR+ breast cancer. Clinical trials
in postmenopausal women have demonstrated a small
advantage to including an AI initially or over the course
of treatment rather than 5 years of tamoxifen alone.
248
Treatment guidelines recommend AIs should usually be
included in the treatment of postmenopausal women with
HR+ breast cancer.
247
Although AIs have fewer serious
side effects than tamoxifen, they can cause osteoporosis
(with resulting bone fractures), joint pain, and other
musculoskeletal symptoms because they completely
deplete postmenopausal women of estrogen. Clinical
trials continue to assess the optimal timing and duration
of these treatments.
The mainstay of treatment for most premenopausal
women with HR+ tumors is tamoxifen. Some women may
also benefit from surgical removal (oophorectomy) or
chemical suppression of the ovaries, which are the main
source of estrogen prior to menopause. Potentially
reversible ovarian suppression can be achieved with a
class of drugs called luteinizing hormone-releasing
hormone (LHRH) analogs. Ovarian suppression can also

28 Breast Cancer Facts & Figures 2017-2018
allow the use of AIs in premenopausal women. Initial
results from two ongoing clinical trials comparing
premenopausal early-stage breast cancer patients treated
with ovarian suppression, plus either an AI or tamoxifen,
found greater reduction in risk of recurrence with AIs.
249
Adding ovarian suppression to tamoxifen or aromatase
inhibitors has been shown to improve survival in
premenopausal women with advanced (metastatic) HR+
breast cancer.
250
Fulvestrant is another treatment used to
treat metastatic breast cancer. It is an anti-estrogen drug
given by intramuscular injection that reduces the number
of estrogen receptors and blocks estrogen binding.
Targeted therapy
About 17% of breast cancers overproduce the growth-
promoting protein HER2/neu, and multiple medications
are now approved for the treatment of this subtype.
Trastuzumab, the first approved drug, is a monoclonal
antibody that directly targets the HER2 protein. The
combined results of two large trials indicate that adding
trastuzumab to standard chemotherapy for early-stage
HER2+ breast cancer reduces the risk of recurrence and
death by 52% and 33%, respectively, compared to
chemotherapy alone.
251
This drug is also a standard part
of the treatment for advanced HER2+ breast cancer.
Several newer drugs have been developed that target the
HER2 protein that can be used in combination with
trastuzumab or if trastuzumab is no longer working. All
invasive breast cancers should be tested for the HER2
gene amplification or protein overexpression to identify
women who would benefit from this therapy.
Other types of targeted therapies can be used along with
aromatase inhibitors in women with HR+ breast cancer,
where they have been shown to make these hormone
therapies more effective.
What Is the American Cancer Society
Doing About Breast Cancer?
As an organization of nearly 2 million strong, the American
Cancer Society is committed to a world free from the
pain and suffering of breast cancer – and all cancers.
Prevention, Early Detection,
and Treatment
The American Cancer Society is doing everything in our
power to help prevent breast cancer – and all cancers. We
promote healthy lifestyles by issuing cancer guidelines
for prevention and early detection, helping people avoid
tobacco, and reducing barriers to healthy eating and
exercise. For those who are diagnosed, we’re there every
minute of every day.
Information, 24 hours a day, seven days a week
The American Cancer Society is available 24 hours a day,
seven days a week online at
cancer.org
and by calling us at
1-800-227-2345. Callers are connected with caring,
trained American Cancer Society staff who can help
them locate a hospital, understand breast cancer and
treatment options, learn what to expect and how to plan,
address insurance concerns, find financial resources,
find a local support group, and more. We can also help
people who speak languages other than English or
Spanish find the assistance they need, offering services in
more than 200 languages.
People can visit
cancer.org/breastcancer
to find information
on every aspect of the breast cancer experience, from
prevention to survivorship. We also publish a wide variety
of pamphlets and books that cover a multitude of topics,
from patient education, quality-of-life and caregiving
issues to healthy living. Visit
cancer.org/bookstore
for a
complete list of books that are available for order.
Breast Cancer Facts & Figures 2017-2018 29
Help navigating the health care system
Learning how to navigate the cancer journey and the
health care system can be overwhelming for anyone, but
it is particularly difficult for those who are medically
underserved, those who experience language or health
literacy barriers, and those with limited resources. The
American Cancer Society Patient Navigator Program
reaches those most in need. The largest oncology-focused
patient navigator program in the country, it has specially
trained patient navigators at more than 120 sites across
the nation. Patient navigators can help: find rides to and
from cancer-related appointments; assist with medical
financial issues, including insurance navigation; identify
community resources; and provide information on a
patient’s cancer diagnosis and treatment process. We
collaborate with a variety of organizations, including the
National Cancer Institute’s Center to Reduce Cancer
Health Disparities, the Center for Medicare and Medicaid
Services, and numerous cancer treatment centers to
implement and evaluate this program.
Breast cancer support
Through the American Cancer Society Reach To
Recovery® program, breast cancer patients are paired
with trained volunteers who have had similar diagnoses
and treatment plans to provide more personal, one-on-
one support.
Finding hope and inspiration
Women with breast cancer and their loved ones do not
have to face their experience alone. The American Cancer
Society Cancer Survivors Network® provides a safe online
connection where cancer patients can find others with
similar experiences and interests. At
csn.cancer.org
,
members can join chat rooms and build their own
support network from among the members.
Transportation to treatment
The American Cancer Society Road To Recovery®
program offers cancer patients free transportation to and
from their cancer-related treatment. For those who
cannot drive themselves or have no other means of
getting to treatment, trained volunteers donate their
spare time and the use of their personal vehicle to give
cancer patients in their community a much-needed ride.
Other transportation programs are also available in
certain areas. Call us at 1-800-227-2345 for more
information.
Lodging during treatment
The American Cancer Society Hope Lodge® program
provides a free home away from home for cancer patients
and their caregivers. More than just a roof over their
heads, it is a nurturing community where patients can
share stories and offer each other emotional support.
Through our Hotel Partners Program, we also partner
with local hotels across the country to provide free or
discounted lodging to patients and their caregivers in
communities without a Hope Lodge facility.
Help with appearance-related side effects
of treatment
The Look Good Feel Better® program teaches women how
to cope with appearance-related side effects of cancer
treatment. Group workshops are free and led by licensed
volunteer beauty professionals (cosmetologists,
estheticians, and nail technicians). Skin care, makeup,
and hair loss solution techniques and tips are provided in
a supportive environment. Information and materials are
also available for men and teens. This program is a
collaboration of the American Cancer Society, the Look
Good Feel Better Foundation, and the Professional
Beauty Association. To learn more, visit the Look Good
Feel Better website at
lookgoodfeelbetter.org
or call 1-800-
395-LOOK (1-800-395-5665).
Hair-loss and mastectomy products
Some women wear wigs, hats, breast forms, and special
bras to help cope with the effects of a mastectomy and
hair loss. The American Cancer Society “tlc” Tender
Loving Care® publication offers affordable hair loss and
mastectomy products, as well as advice on how to use
those products. The “tlc”
TM
products and catalogs may be
ordered online at
tlcdirect.org
or by calling 1-800-850-
9445. All proceeds from product sales go back into our
survivorship programs and services.
30 Breast Cancer Facts & Figures 2017-2018
Support after treatment
The end of breast cancer treatment does not mean the
end of a cancer journey. Cancer survivors may experience
long-term or late effects resulting from the disease or its
treatment. The Life After Treatment: The Next Chapter in
Your Survivorship Journey guide may help cancer
survivors as they begin the next phase of their journey.
Visit
cancer.org/survivorshipguide
to download a free copy
of the guide.
The American Cancer Society has also recently published
a follow-up care guideline for breast cancer survivors
that builds upon available evidence, surveillance
guidelines, and standard clinical practice and is designed
to facilitate the provision of high-quality, standardized,
clinical care by primary care providers.
252
The breast
cancer guideline addresses the assessment and
management of potential long-term and late effects, as
well as recommendations for health promotion,
surveillance for recurrence, screening for second primary
cancers, and the coordination of care between specialists
and primary care clinicians.
Research
The American Cancer Society invests more in breast
cancer research than any other cancer type. Our funded
research has led to the development of potentially
lifesaving breast cancer drugs such as tamoxifen and
Herceptin, as well as improved understanding of genes
linked to breast cancer. We are currently funding more
than $59 million in breast cancer research through 159
research and training grants. These grants are awarded
in multiple areas relevant to the disease, including
genetics, etiology, diagnostics (imaging and biomarkers),
drug development; and preclinical, clinical, and
epidemiological studies in prevention, diagnosis,
treatment, and quality of life.
Specific examples of ongoing breast cancer research
being conducted by American Cancer Society grantees
include:
• Researching new ways of treating HER2+ breast
cancer patients who do not respond to or become
resistant to existing targeted therapies
• Evaluating psychosocial interventions aimed at
supporting Latinas with breast cancer and their
family partners to reduce distress and improve
quality of life
• Exploring how a gene called amphiregulin may cause
a woman with ER+ breast cancer to become resistant
to hormonal therapies
• Investigating ways to prevent breast cancer patients
from developing brain metastases by studying
proteins that may be involved in the spread of breast
cancer to the brain
• Evaluating whether a non-invasive and inexpensive
technique called auricular point acupressure can
help women with breast cancer manage their pain
at home
Internally, the American Cancer Society also conducts
epidemiologic studies of breast cancer and performs
surveillance research to monitor racial and
socioeconomic disparities in breast cancer screening,
incidence, survival, and mortality. Using information
collected from more than 600,000 women in Cancer
Prevention Study-II (CPS-II), American Cancer Society
epidemiologists study the influence of many risk factors,
including alcohol consumption, diethylstilbestrol (DES),
estrogen hormone use, family history of cancer, obesity,
smoking, and spontaneous abortion on the risk of death
from breast cancer. In order to continue to explore the
effects of changing exposures and to provide greater
opportunity to integrate biological and genetic factors
into studies of other risk factors, more than 304,000 men
and women were enrolled in the American Cancer
Society Cancer Prevention Study-3 (CPS-3), and nearly all
provided a blood sample at the time of enrollment. The
blood specimens and questionnaire data collected from
CPS-3 participants will provide unique opportunities for
research in the US.
Advocacy
The American Cancer Society’s nonprofit, nonpartisan
advocacy affiliate, the American Cancer Society Cancer
Action Network
SM
(ACS CAN), advocates at the federal,
state, and local levels to increase access to quality breast
Breast Cancer Facts & Figures 2017-2018 31
cancer screenings, diagnostic and treatment services, and
care for all women; increase government funding for breast
cancer research; and provide a voice for the concerns of
breast cancer patients and survivors. Following are some
of the efforts that ACS CAN has been involved with in the
past few years to fight breast cancer – and all cancers:
• Improving Access to Affordable Care through
Health Care Reform: The Affordable Care Act (ACA)
was signed into law on March 23, 2010, giving cancer
patients access to quality, affordable health care. All
new health insurance plans, including those offered
through state health insurance exchanges, are
required to cover preventive services rated “A” or “B”
by the US Preventive Services Task Force, including
mammography screening, at no cost to patients.
Additionally, the ACA removed cost sharing for any
preventive services covered by Medicare. ACS CAN
advocates for clear, comprehensive coverage of these
preventive services, including breast cancer
screening, and encourages states to broaden access
to health care coverage for all low-income Americans
through state Medicaid programs.
• The National Breast and Cervical Cancer Early
Detection Program (NBCCEDP): Protecting and
increasing funding for the NBCCEDP is a high priority
for ACS CAN at both the state and federal levels.
Administered by the Centers for Disease Control
and Prevention, this successful program provides
community-based breast and cervical cancer
screenings to low-income, uninsured, and underinsured
women. More than 50% of the women screened are from
racial/ethnic minority groups. Currently, only one in
10 eligible women can be served by the program due
to federal funding cuts. ACS CAN is asking Congress
to increase funding to ensure that more women have
access to cancer screening.
• Protecting the Breast and Cervical Cancer
Prevention and Treatment Act (BCCPTA): In 2000,
Congress passed the BCCPTA, ensuring that low-
income women diagnosed with cancer through the
NBCCEDP were provided a pathway to treatment
services through their state Medicaid program.
In recent years, a number of states have considered
proposals to eliminate the treatment program due to
misconceptions around coverage needs following
implementation of the ACA.
• The Breast Density and Mammography Reporting
Act: Mammography sensitivity is lower for women
with mammographically dense breasts because dense
breast tissue makes it harder for doctors to see cancer
on mammograms. The federal Breast Density and
Mammography Reporting Act directs an evidence-
based process to inform women about breast density
and risk. Additionally, this legislation encourages
new research to support the creation of clinical
guidelines and best practices for screening of and
reports to women with mammographically dense
breasts.
• Patient Navigation: Patient navigation can improve
quality of cancer care, particularly in vulnerable
populations. ACS CAN supports the federal Patient
Navigation Assistance Act, which would create a
coverage solution that incentivizes providers to
use patient navigators in order to improve care
coordination for patients. The organization also is
working with Congress and federal agencies to help
increase funding for patient navigation programs.
• Funding for Cancer Research: ACS CAN continues
to work to increase government funding for cancer
research at the National Institutes of Health, including
the National Cancer Institute and the National
Center on Minority Health and Health Disparities.
It is important to note that the preceding references to
ACA provisions and other federal laws and guidance
reflect current law as of July 18, 2017, and do not take into
account potential changes to the ACA or other federal
laws and guidance subsequently considered by Congress
and the administration.

32 Breast Cancer Facts & Figures 2017-2018
Sources of Statistics
General information. Unless otherwise stated, the
statistics and statements in this booklet refer to invasive
(not in situ) female breast cancer.
Estimated new breast cancer cases. The overall
estimated number of new in situ and invasive breast
cancer cases diagnosed in the US in 2017 was projected
using a spatiotemporal model based on incidence data
from 49 states and the District of Columbia for the years
1995-2013 that met the North American Association of
Central Cancer Registries’ (NAACCR) high-quality data
standard for incidence. This method considers
geographic variations in sociodemographic and lifestyle
factors, medical settings, and cancer screening behaviors
as predictors of incidence, and also accounts for expected
delays in case reporting. Estimates for specific age groups
are based on the proportions of cases diagnosed in each
age group in the NAACCR data during 2010-2014 applied
to the overall 2017 estimate.
Incidence rates. Incidence rates are defined as the
number of people per 100,000 who develop a disease
during a given time period. All incidence rates in this
publication are age adjusted to the 2000 US standard
population to allow comparisons across populations with
different age distributions. Breast cancer incidence rates
for the US in the most recent time period were calculated
using data on cancer cases collected by NAACCR and
population data collected by the US Census Bureau.
When referenced as such, NAACCR incidence data were
made available on the NAACCR website (
naaccr.org
) and
within the Cancer in North America publications.
253, 254
Long-term incidence trends are based on American
Cancer Society analysis of the SEER 9 Registries Public
Use Dataset using SEER*Stat 8.3.4, a statistical software
package from the National Cancer Institute.
255, 256
Short-
term trends by race/ethnicity, age, tumor size, and stage
at diagnosis are based on delay-adjusted incidence rates
from the SEER 13 registries.
22
When referenced as such,
US SEER incidence rates and trends were previously
made available on SEER’s website (seer.cancer. gov) and
within the SEER Cancer Statistics Review 1975-2014.
17
Note that because of delays in reporting newly diagnosed
cancer cases to the cancer registries, cancer incidence rates
for the most recent diagnosis years may be underestimated.
Incidence rates adjusted for delays in reporting are used
when available and are referenced as such.
Estimated breast cancer deaths. The overall estimated
number of breast cancer deaths in the US is calculated by
fitting the number of breast cancer deaths for 1997-2014
to a statistical model that forecasts the number of deaths
expected to occur in 2017. Data on the number of deaths
are obtained from the National Center for Health
Statistics (NCHS) at the Centers for Disease Control and
Prevention. Age-specific estimates were calculated using
the proportions of deaths that occurred in each age group
during 2011-2015 applied to the overall 2017 estimate.
Mortality rates. Similar to incidence rates, mortality
rates are defined as the number of people per 100,000
who die from a disease during a given time period. Death
rates used in this publication were previously made
available by SEER on their website (
seer.cancer.gov
) and
within the SEER Cancer Statistics Review 1975-2014.
17
Death rates were calculated using data on cancer deaths
compiled by NCHS and population data collected by the
US Census Bureau. All death rates in this publication
were age adjusted to the 2000 US standard population.
Survival. Five-year survival statistics are based on
cancer patients diagnosed during 2007-2013; 10-year
survival rates are based on diagnoses during 2001-2013;
and 15-year survival rates are based on diagnoses during
1996-2013. All patients were followed through 2014.
Relative survival rates are used to adjust for normal life
expectancy (and events such as death from heart disease,
accidents, and diseases of old age). Relative survival is
calculated by dividing the percentage of observed 5-year
survival for cancer patients by the 5-year survival
expected for people in the general population who are
similar to the patient group with respect to age, sex, race,
and calendar year of observation. Cause-specific survival
rates are the probability of not dying of breast cancer

Breast Cancer Facts & Figures 2017-2018 33
within 5 years after diagnosis. When referenced as such,
5-year survival statistics were originally published in
SEER Cancer Statistics Review, 1975-2014.
17
Probability of developing cancer. Probabilities of
developing breast cancer were calculated using DevCan
6.7.5 (Probability of Developing Cancer Software),
developed by the National Cancer Institute.
257
These
probabilities reflect the average experience of women in
the US and do not take into account individual behaviors
and risk factors (e.g., utilization of mammography
screening and family history of breast cancer).
Screening. Prevalence estimates of mammography by
age and state were obtained through analysis of data
from the Behavioral Risk Factor Surveillance System
(BRFSS).
196
The BRFSS is an ongoing system of surveys
conducted by the state health departments in
cooperation with the Centers for Disease Control and
Prevention. Prevalence estimates of mammography by
race/ethnicity, poverty, and other demographic factors
are from the National Health Interview Survey.
195
Important note about estimated cases and deaths. The
estimated numbers of new breast cancer cases and
deaths in 2017 should be interpreted with caution. The
projection method is model-based, so the estimated
numbers may vary from previous years for reasons other
than changes in cancer occurrence. Therefore, while
3-year-ahead projections provide a reasonably accurate
estimate of the cancer burden in 2017, we strongly
discourage the use of our estimates to track changes in
cancer occurrence. Age-adjusted incidence and mortality
rates reported by the SEER program and the NCHS,
respectively, are the preferred statistics to track cancer
trends in the US. Rates from state cancer registries are
useful for tracking local trends.
References
1. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FG, Trotti A. AJCC
Cancer Staging Manual. New York: Springer, 2010.
2. Giuliano AE, Connolly JL, Edge SB, et al. Breast Cancer – Major
changes in the American Joint Committee on Cancer eighth edition
cancer staging manual. CA Cancer J Clin. 2017;67: 290-303.
3. Allred DC. Ductal carcinoma in situ: terminology, classification,
and natural history. J Natl Cancer Inst Monogr. 2010;2010: 134-138.
4. Erbas B, Provenzano E, Armes J, Gertig D. The natural history of
ductal carcinoma in situ of the breast: a review. Breast Cancer Res
Treat. 2006;97: 135-144.
5. Sanders ME, Schuyler PA, Dupont WD, Page DL. The natural
history of low-grade ductal carcinoma in situ of the breast in women
treated by biopsy only revealed over 30 years of long-term follow-up.
Cancer. 2005;103: 2481-2484.
6. Collins LC, Tamimi RM, Baer HJ, Connolly JL, Colditz GA, Schnitt
SJ. Outcome of patients with ductal carcinoma in situ untreated after
diagnostic biopsy: results from the Nurses’ Health Study. Cancer.
2005;103: 1778-1784.
7. Eusebi V, Feudale E, Foschini MP, et al. Long-term follow-up of in situ
carcinoma of the breast. Seminars in Diagn Path. 1994;11: 223-235.
8. Dieci MV, Orvieto E, Dominici M, Conte P, Guarneri V. Rare breast
cancer subtypes: histological, molecular, and clinical peculiarities.
Oncologist. 2014;19: 805-813.
9. Tamimi RM, Colditz GA, Hazra A, et al. Traditional breast cancer
risk factors in relation to molecular subtypes of breast cancer. Breast
Cancer Res Treat. 2012;131: 159-167.
10. Cancer Genome Atlas Network. Comprehensive molecular
portraits of human breast tumours. Nature. 2012;490: 61-70.
11. Cheang MC, Martin M, Nielsen TO, et al. Defining breast cancer
intrinsic subtypes by quantitative receptor expression. Oncologist.
2015;20: 474-482.
12. Blows FM, Driver KE, Schmidt MK, et al. Subtyping of breast
cancer by immunohistochemistry to investigate a relationship
between subtype and short and long term survival: a collaborative
analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010;7:
e1000279.
13. Haque R, Ahmed SA, Inzhakova G, et al. Impact of breast cancer
subtypes and treatment on survival: an analysis spanning two
decades. Cancer Epidemiol Biomarkers Prev. 2012;21: 1848-1855.
14. Perou CM, Borresen-Dale AL. Systems biology and genomics of
breast cancer. Cold Spring Harb Perspect Biol. 2011;3.
15. Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L.
Triple-negative breast cancer: challenges and opportunities of a
heterogeneous disease. Nature Rev Clin Oncol. 2016;13: 674-690.
16. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and
survivorship statistics, 2016. CA Cancer J Clin. 2016;66: 271-289.
17. Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics
Review, 1975-2014.
https://seer.cancer.gov/csr/1975_2014/
, based on
November 2016 SEER data submission, posted to the SEER web site,
April 2017. Bethesda, MD: National Cancer Institute, 2017.
18. White MC, Espey DK, Swan J, Wiggins CL, Eheman C, Kaur JS.
Disparities in cancer mortality and incidence among American
Indians and Alaska Natives in the United States. Amer J Pub Health.
2014;104 Suppl 3: S377-387.
34 Breast Cancer Facts & Figures 2017-2018
19. Ravdin PM, Cronin KA, Howlader N, et al. The decrease in breast
cancer incidence in 2003 in the United States. N Engl J Med. 2007;356:
1670-1674.
20. Coombs NJ, Cronin KA, Taylor RJ, Freedman AN, Boyages J. The
impact of changes in hormone therapy on breast cancer incidence in
the US population. Cancer Causes Control. 2010;21: 83-90.
21. DeSantis C, Howlader N, Cronin KA, Jemal A. Breast cancer
incidence rates in U.S. women are no longer declining. Cancer
Epidemiol Biomarkers Prev. 2011;20: 733-739.
22. Surveillance, Epidemiology, and End Results (SEER) Program
(
www.seer.cancer.gov
) SEER*Stat Database: Incidence – SEER 13 Regs
Research Data with Delay-Adjustment, Malignant Only, Nov 2016
Sub (1992-2014) <Katrina/Rita Population Adjustment> – Linked To
County Attributes – Total U.S., 1969-2015 Counties, National Cancer
Institute, DCCPS, Surveillance Research Program, released April
2017, based on the November 2016 submission.
23. Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and
adjuvant therapy on mortality from breast cancer. N Engl J Med.
2005;353: 1784-1792.
24. Newman LA, Kaljee LM. Health Disparities and Triple-Negative
Breast Cancer in African American Women: A Review. JAMA Surg. 2017.
25. Roberts MC, Wheeler SB, Reeder-Hayes K. Racial/Ethnic and
socioeconomic disparities in endocrine therapy adherence in breast
cancer: a systematic review. Amer J Public Health. 2015;105 Suppl 3:
e4-e15.
26. Iqbal J, Ginsburg O, Rochon PA, Sun P, Narod SA. Differences in
breast cancer stage at diagnosis and cancer-specific survival by race
and ethnicity in the United States. JAMA. 2015;313: 165-173.
27. Daly B, Olopade OI. A perfect storm: How tumor biology,
genomics, and health care delivery patterns collide to create a racial
survival disparity in breast cancer and proposed interventions for
change. CA Cancer J Clin. 2015;65: 221-238.
28. Allgood KL, Rauscher GH, Whitman S, Vasquez-Jones G, Shah
AM. Validating self-reported mammography use in vulnerable
communities: findings and recommendations. Cancer Epidemiol
Biomarkers Prev. 2014;23: 1649-1658.
29. Njai R, Siegel PZ, Miller JW, Liao Y. Misclassification of survey
responses and black-white disparity in mammography use,
Behavioral Risk Factor Surveillance System, 1995-2006. Prev Chronic
Dis. 2011;8: A59.
30. Cronin KA, Miglioretti DL, Krapcho M, et al. Bias associated
with self-report of prior screening mammography. Cancer Epidemiol
Biomarkers Prev. 2009;18: 1699-1705.
31. Surveillance, Epidemiology, and End Results (SEER) Program
(
www.seer.cancer.gov
) SEER*Stat Database: Incidence – SEER 18 Regs
Research Data, Nov 2016 Sub (2000-2014) <Katrina/Rita Population
Adjustment> – Linked To County Attributes – Total U.S., 1969-
2015 Counties, National Cancer Institute, DCCPS, Surveillance
Research Program, released April 2017, based on the November 2016
submission.
32. Keegan TH, Kurian AW, Gali K, et al. Racial/ethnic and
socioeconomic differences in short-term breast cancer survival
among women in an integrated health system. Amer J Public Health.
2015;105: 938-946.
33. Shariff-Marco S, Yang J, John EM, et al. Impact of neighborhood
and individual socioeconomic status on survival after breast cancer
varies by race/ethnicity: the Neighborhood and Breast Cancer Study.
Cancer Epidemiol Biomarkers Prev. 2014;23: 793-811.
34. Shi R, Taylor H, McLarty J, Liu L, Mills G, Burton G. Effects of
payer status on breast cancer survival: a retrospective study. BMC
Cancer. 2015;15: 211.
35. Kish JK, Yu M, Percy-Laurry A, Altekruse SF. Racial and ethnic
disparities in cancer survival by neighborhood socioeconomic status
in Surveillance, Epidemiology, and End Results (SEER) Registries. J
Natl Cancer Inst Monogr. 2014;2014: 236-243.
36. Sprague BL, Trentham-Dietz A, Gangnon RE, et al. Socioeconomic
status and survival after an invasive breast cancer diagnosis. Cancer.
2011;117: 1542-1551.
37. Anderson WF, Jatoi I, Tse J, Rosenberg PS. Male breast cancer: a
population-based comparison with female breast cancer. J Clin Oncol.
2010;28: 232-239.
38. Brinton LA, Cook MB, McCormack V, et al. Anthropometric and
hormonal risk factors for male breast cancer: male breast cancer
pooling project results. J Natl Cancer Inst. 2014;106: djt465.
39. Ruddy KJ, Winer EP. Male breast cancer: risk factors, biology,
diagnosis, treatment, and survivorship. Ann Oncol. 2013;24: 1434-1443.
40. Tamimi RM, Spiegelman D, Smith-Warner SA, et al. Population
Attributable Risk of Modifiable and Nonmodifiable Breast Cancer
Risk Factors in Postmenopausal Breast Cancer. Am J Epidemiol.
2016;184: 884-893.
41. Liu Y, Nguyen N, Colditz GA. Links between alcohol consumption
and breast cancer: a look at the evidence. Women’s Health (London,
England). 2015;11: 65-77.
42. Dall GV, Britt KL. Estrogen Effects on the Mammary Gland in
Early and Late Life and Breast Cancer Risk. Front Oncol. 2017;7: 110
43. Barnard ME, Boeke CE, Tamimi RM. Established breast cancer
risk factors and risk of intrinsic tumor subtypes. Biochim Biophys
Acta. 2015;1856: 73-85.
44. Collaborative Group on Hormonal Factors in Breast Cancer.
Familial breast cancer: collaborative reanalysis of individual data
from 52 epidemiological studies including 58,209 women with breast
cancer and 101,986 women without the disease. Lancet. 2001;358:
1389-1399.
45. Turnbull C, Rahman N. Genetic predisposition to breast cancer:
past, present, and future. Ann Rev Genomics Hum Genet. 2008;9: 321-
345.
46. Tung N, Lin NU, Kidd J, et al. Frequency of Germline Mutations in
25 Cancer Susceptibility Genes in a Sequential Series of Patients With
Breast Cancer. J Clin Oncol. 2016;34: 1460-1468.
47. Gabai-Kapara E, Lahad A, Kaufman B, et al. Population-based
screening for breast and ovarian cancer risk due to BRCA1 and
BRCA2. Proc Natl Acad Sci U S A. 2014;111: 14205-14210.
48. Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of Breast,
Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2
Mutation Carriers. JAMA. 2017;317: 2402-2416.
49. Antoniou AC, Casadei S, Heikkinen T, et al. Breast-cancer risk in
families with mutations in PALB2. N Engl J Med. 2014;371: 497-506.
50. Michailidou K, Beesley J, Lindstrom S, et al. Genome-wide
association analysis of more than 120,000 individuals identifies 15
new susceptibility loci for breast cancer. Nature Genetics. 2015;47:
373-380.
51. Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and
heritable factors in the causation of cancer – analyses of cohorts of
twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343:
78-85.
Breast Cancer Facts & Figures 2017-2018 35
52. Moyer VA. Risk assessment, genetic counseling, and genetic
testing for BRCA-related cancer in women: U.S. Preventive Services
Task Force recommendation statement. Ann Intern Med. 2014;160:
271-281.
53. Nichols HB, Berrington de Gonzalez A, Lacey JV, Jr., Rosenberg PS,
Anderson WF. Declining incidence of contralateral breast cancer in
the United States from 1975 to 2006. J Clin Oncol. 2011;29: 1564-1569.
54. Gierach GL, Curtis RE, Pfeiffer RM, et al. Association of Adjuvant
Tamoxifen and Aromatase Inhibitor Therapy With Contralateral
Breast Cancer Risk Among US Women With Breast Cancer in a
General Community Setting. JAMA Oncol. 2016.
55. Lopez-Garcia MA, Geyer FC, Lacroix-Triki M, Marchio C, Reis-
Filho JS. Breast cancer precursors revisited: molecular features and
progression pathways. Histopathology. 2010;57: 171-192.
56. Morrow M, Schnitt SJ, Norton L. Current management of lesions
associated with an increased risk of breast cancer. Nat Rev Clin Oncol.
2015;12: 227-238.
57. King TA, Pilewskie M, Muhsen S, et al. Lobular Carcinoma in Situ:
A 29-Year Longitudinal Experience Evaluating Clinicopathologic
Features and Breast Cancer Risk. J Clin Oncol. 2015;33: 3945-3952.
58. Dyrstad SW, Yan Y, Fowler AM, Colditz GA. Breast cancer risk
associated with benign breast disease: systematic review and meta-
analysis. Breast Cancer Res Treat. 2015;149: 569-575.
59. Bertrand KA, Scott CG, Tamimi RM, et al. Dense and nondense
mammographic area and risk of breast cancer by age and tumor
characteristics. Cancer Epidemiol Biomarkers Prev. 2015;24: 798-809.
60. Sprague BL, Gangnon RE, Burt V, et al. Prevalence of
mammographically dense breasts in the United States. J Natl Cancer
Inst. 2014;106.
61. Boyd NF, Martin LJ, Rommens JM, et al. Mammographic density:
a heritable risk factor for breast cancer. Methods Mol Biol. 2009;472:
343-360.
62. Harris HR, Tamimi RM, Willett WC, Hankinson SE, Michels KB.
Body size across the life course, mammographic density, and risk of
breast cancer. Am J Epidemiol. 2011;174: 909-918.
63. Boyd NF. Tamoxifen, mammographic density, and breast cancer
prevention. J Natl Cancer Inst. 2011;103: 704-705.
64. Byrne C, Ursin G, Martin CF, et al. Mammographic Density
Change With Estrogen and Progestin Therapy and Breast Cancer
Risk. J Natl Cancer Inst. 2017;109.
65. Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the
risk and detection of breast cancer. N Engl J Med. 2007;356: 227-236.
66. DenseBreast-Info, Inc. LEGISLATION AND REGULATIONS –
WHAT IS REQUIRED? Available from URL:
densebreast-info.org/legislation
Accessed August 22, 2017.
67. Melnikow J, Fenton JJ, Whitlock EP, et al. Supplemental Screening
for Breast Cancer in Women with Dense Breasts: A Systematic Review
for the U.S. Preventive Services Task Force2016, Ann Intern Med.
2016;164: 268-278.
68. Trubo R. Recent findings may inform breast density notification
laws. JAMA. 2015;313: 452-453.
69. van den Brandt PA, Spiegelman D, Yaun SS, et al. Pooled analysis
of prospective cohort studies on height, weight, and breast cancer
risk. Am J Epidemiol. 2000;152: 514-527.
70. Green J, Cairns BJ, Casabonne D, Wright FL, Reeves G, Beral
V. Height and cancer incidence in the Million Women Study:
prospective cohort, and meta-analysis of prospective studies of
height and total cancer risk. Lancet Oncol. 2011;12: 785-794.
71. Wiren S, Haggstrom C, Ulmer H, et al. Pooled cohort study on
height and risk of cancer and cancer death. Cancer Causes Control.
2014;25: 151-159.
72. Collaborative Group on Hormonal Factors in Breast Cancer.
Menarche, menopause, and breast cancer risk: individual participant
meta-analysis, including 118,964 women with breast cancer from 117
epidemiological studies. Lancet Oncol. 2012;13: 1141-1151.
73. Anderson KN, Schwab RB, Martinez ME. Reproductive risk
factors and breast cancer subtypes: a review of the literature. Breast
Cancer Res Treat. 2014;144: 1-10.
74. Qu X, Zhang X, Qin A, et al. Bone mineral density and risk of
breast cancer in postmenopausal women. Breast Cancer Res Treat.
2013;138: 261-271.
75. Grenier D, Cooke AL, Lix L, Metge C, Lu H, Leslie WD. Bone
mineral density and risk of postmenopausal breast cancer. Breast
Cancer Res Treat. 2011;126: 679-686.
76. Kerlikowske K, Shepherd J, Creasman J, Tice JA, Ziv E, Cummings
SR. Are breast density and bone mineral density independent risk
factors for breast cancer? J Natl Cancer Inst. 2005;97: 368-374.
77. Sampson JN, Falk RT, Schairer C, et al. Association of Estrogen
Metabolism with Breast Cancer Risk in Different Cohorts of
Postmenopausal Women. Cancer Res. 2017;77: 918-925.
78. Endogenous Hormones Breast Cancer Collaborative Group, Key
TJ, Appleby PN, et al. Circulating sex hormones and breast cancer
risk factors in postmenopausal women: reanalysis of 13 studies. Br J
Cancer. 2011;105: 709-722.
79. Key TJ, Appleby PN, Reeves GK, et al. Sex hormones and risk of
breast cancer in premenopausal women: a collaborative reanalysis
of individual participant data from seven prospective studies. Lancet
Oncol. 2013;14: 1009-1019.
80. Lambertini M, Santoro L, Del Mastro L, et al. Reproductive
behaviors and risk of developing breast cancer according to tumor
subtype: A systematic review and meta-analysis of epidemiological
studies. Cancer Treat Rev. 2016;49: 65-76.
81. Albrektsen G, Heuch I, Hansen S, Kvale G. Breast cancer risk by
age at birth, time since birth and time intervals between births:
exploring interaction effects. Br J Cancer. 2005;92: 167-175.
82. Schedin P. Pregnancy-associated breast cancer and metastasis.
Nature Rev Cancer. 2006;6: 281-291.
83. Brinton LA. Fertility Status and Cancer. Semin Reprod Med.
2017;35: 291-297.
84. Brinton LA, Scoccia B, Moghissi KS, et al. Long-term relationship
of ovulation-stimulating drugs to breast cancer risk. Cancer
Epidemiol Biomarkers Prev. 2014;23: 584-593.
85. van den Belt-Dusebout AW, Spaan M, Lambalk CB, et al. Ovarian
Stimulation for In Vitro Fertilization and Long-term Risk of Breast
Cancer. JAMA. 2016;316: 300-312.
86. Faupel-Badger JM, Arcaro KF, Balkam JJ, et al. Postpartum
remodeling, lactation, and breast cancer risk: summary of a National
Cancer Institute-sponsored workshop. J Natl Cancer Inst. 2013;105:
166-174.
87. Collaborative Group on Hormonal Factors in Breast Cancer.
Breast cancer and breastfeeding: collaborative reanalysis of
individual data from 47 epidemiological studies in 30 countries,
including 50,302 women with breast cancer and 96,973 women
without the disease. Lancet. 2002;360: 187-195.
88. Britt K, Ashworth A, Smalley M. Pregnancy and the risk of breast
cancer. Endocrine-related Cancer. 2007;14: 907-933.
36 Breast Cancer Facts & Figures 2017-2018
89. Sisti JS, Collins LC, Beck AH, Tamimi RM, Rosner BA, Eliassen AH.
Reproductive risk factors in relation to molecular subtypes of breast
cancer: Results from the nurses’ health studies. Int J Cancer. 2016;138:
2346-2356.
90. Islami F, Liu Y, Jemal A, et al. Breastfeeding and breast cancer
risk by receptor status – a systematic review and meta-analysis. Ann
Oncol. 2015;26: 2398-2407.
91. Bassuk SS, Manson JE. Oral contraceptives and menopausal
hormone therapy: relative and attributable risks of cardiovascular
disease, cancer, and other health outcomes. Ann Epidemiol. 2015;25:
193-200.
92. Li CI, Beaber EF, Tang MT, Porter PL, Daling JR, Malone KE. Effect
of depo-medroxyprogesterone acetate on breast cancer risk among
women 20 to 44 years of age. Cancer Res. 2012;72: 2028-2035.
93. Shantakumar S, Terry MB, Paykin A, et al. Age and menopausal
effects of hormonal birth control and hormone replacement therapy
in relation to breast cancer risk. Am J Epidemiol. 2007;165: 1187-1198.
94. Strom BL, Berlin JA, Weber AL, et al. Absence of an effect of
injectable and implantable progestin-only contraceptives on
subsequent risk of breast cancer. Contraception. 2004;69: 353-360.
95. Ellingjord-Dale M, Vos L, Tretli S, Hofvind S, Dos-Santos-Silva I,
Ursin G. Parity, hormones and breast cancer subtypes – results from
a large nested case-control study in a national screening program.
Breast Cancer Res. 2017;19: 10.
96. Soini T, Hurskainen R, Grenman S, Maenpaa J, Paavonen J,
Pukkala E. Cancer risk in women using the levonorgestrel-releasing
intrauterine system in Finland. Obstet Gynecol. 2014;124: 292-299.
97. Dinger J, Bardenheuer K, Minh TD. Levonorgestrel-releasing
and copper intrauterine devices and the risk of breast cancer.
Contraception. 2011;83: 211-217.
98. Trinh XB, Tjalma WA, Makar AP, Buytaert G, Weyler J, van Dam
PA. Use of the levonorgestrel-releasing intrauterine system in breast
cancer patients. Fertil Steril. 2008;90: 17-22.
99. Chlebowski RT, Manson JE, Anderson GL, et al. Estrogen plus
progestin and breast cancer incidence and mortality in the Women’s
Health Initiative Observational Study. J Natl Cancer Inst. 2013;105:
526-535.
100. Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal
hormone therapy and health outcomes during the intervention and
extended poststopping phases of the Women’s Health Initiative
randomized trials. JAMA. 2013;310: 1353-1368.
101. Beral V, Reeves G, Bull D, Green J. Breast cancer risk in relation
to the interval between menopause and starting hormone therapy. J
Natl Cancer Inst. 2011;103: 296-305.
102. Chlebowski RT, Rohan TE, Manson JE, et al. Breast Cancer After
Use of Estrogen Plus Progestin and Estrogen Alone: Analyses of Data
From 2 Women’s Health Initiative Randomized Clinical Trials. JAMA
Oncol. 2015;1: 296-305.
103. LaCroix AZ, Chlebowski RT, Manson JE, et al. Health outcomes
after stopping conjugated equine estrogens among postmenopausal
women with prior hysterectomy: a randomized controlled trial.
JAMA. 2011;305: 1305-1314.
104. Nelson HD, Walker M, Zakher B, Mitchell J. Menopausal
hormone therapy for the primary prevention of chronic conditions: a
systematic review to update the U.S. Preventive Services Task Force
recommendations. Ann Intern Med. 2012;157: 104-113.
105. Calle EE, Feigelson HS, Hildebrand JS, Teras LR, Thun MJ,
Rodriguez C. Postmenopausal hormone use and breast cancer
associations differ by hormone regimen and histologic subtype.
Cancer. 2009;115: 936-945.
106. Bakken K, Fournier A, Lund E, et al. Menopausal hormone
therapy and breast cancer risk: impact of different treatments. The
European Prospective Investigation into Cancer and Nutrition. Int J
Cancer. 2011;128: 144-156.
107. Chlebowski RT, Anderson GL. The influence of time from
menopause and mammography on hormone therapy-related breast
cancer risk assessment. J Natl Cancer Inst. 2011;103: 284-285.
108. La Vecchia C, Giordano SH, Hortobagyi GN, Chabner B.
Overweight, obesity, diabetes, and risk of breast cancer: interlocking
pieces of the puzzle. Oncologist. 2011;16: 726-729.
109. Gunter MJ, Wang T, Cushman M, et al. Circulating Adipokines
and Inflammatory Markers and Postmenopausal Breast Cancer Risk.
J Natl Cancer Inst. 2015;107.
110. Picon-Ruiz M, Morata-Tarifa C, Friedman ER, Singerland JM.
Obesity and adverse breast cancer risk and outcome: mechanistic
insights and strategies for intervention. CA Cancer J Clin. 2017.
111. Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP.
Type 2 diabetes and cancer: umbrella review of meta-analyses of
observational studies. BMJ. 2015;350: g7607.
112. Maskarinec G, Jacobs S, Park SY, et al. Type II Diabetes, Obesity,
and Breast Cancer Risk: The Multiethnic Cohort. Cancer Epidemiol
Biomarkers Prev. 2017;26: 854-861.
113. Boyle P, Boniol M, Koechlin A, et al. Diabetes and breast cancer
risk: a meta-analysis. Br J Cancer. 2012;107: 1608-1617.
114. Keum N, Greenwood DC, Lee DH, et al. Adult weight gain
and adiposity-related cancers: a dose-response meta-analysis of
prospective observational studies. J Natl Cancer Inst. 2015;107.
115. Emaus MJ, van Gils CH, Bakker MF, et al. Weight change in
middle adulthood and breast cancer risk in the EPIC-PANACEA
study. Int J Cancer. 2014;135: 2887-2899.
116. Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE.
Adult weight change and risk of postmenopausal breast cancer.
JAMA. 2006;296: 193-201.
117. Teras LR, Goodman M, Patel AV, Diver WR, Flanders WD,
Feigelson HS. Weight loss and postmenopausal breast cancer in
a prospective cohort of overweight and obese US women. Cancer
Causes Control. 2011;22: 573-579.
118. Nelson HD, Zakher B, Cantor A, et al. Risk factors for breast
cancer for women aged 40 to 49 years: a systematic review and meta-
analysis. Ann Intern Med. 2012;156: 635-648.
119. Pizot C, Boniol M, Mullie P, et al. Physical activity, hormone
replacement therapy and breast cancer risk: A meta-analysis of
prospective studies. Eur J Cancer. 2016;52: 138-154.
120. Hildebrand JS, Gapstur SM, Campbell PT, Gaudet MM, Patel AV.
Recreational physical activity and leisure-time sitting in relation to
postmenopausal breast cancer risk. Cancer Epidemiol Biomarkers
Prev. 2013;22: 1906-1912.
121. Neilson HK, Friedenreich CM, Brockton NT, Millikan RC.
Physical activity and postmenopausal breast cancer: proposed
biologic mechanisms and areas for future research. Cancer Epidemiol
Biomarkers Prev. 2009;18: 11-27.
122. Cao Y, Hou L, Wang W. Dietary total fat and fatty acids intake,
serum fatty acids and risk of breast cancer: A meta-analysis of
prospective cohort studies. Int J Cancer. 2016;138: 1894-1904.
Breast Cancer Facts & Figures 2017-2018 37
123. Chen M, Rao Y, Zheng Y, et al. Association between soy
isoflavone intake and breast cancer risk for pre- andpost-menopausal
women: a meta-analysis of epidemiological studies. PloS one. 2014;9:
e89288.
124. Farvid MS, Chen WY, Michels KB, Cho E, Willett WC, Eliassen
AH. Fruit and vegetable consumption in adolescence and early
adulthood and risk of breast cancer: population based cohort study.
BMJ. 2016;353: i2343.
125. Emaus MJ, Peeters PH, Bakker MF, et al. Vegetable and fruit
consumption and the risk of hormone receptor-defined breast cancer
in the EPIC cohort. Am J Clin Nutr. 2016;103: 168-177.
126. Jung S, Spiegelman D, Baglietto L, et al. Fruit and vegetable
intake and risk of breast cancer by hormone receptor status. J Natl
Cancer Inst. 2013;105: 219-236.
127. Bakker MF, Peeters PH, Klaasen VM, et al. Plasma carotenoids,
vitamin C, tocopherols, and retinol and the risk of breast cancer in
the European Prospective Investigation into Cancer and Nutrition
cohort. Am J Clin Nutr. 2016;103: 454-464.
128. Eliassen AH, Liao X, Rosner B, Tamimi RM, Tworoger SS,
Hankinson SE. Plasma carotenoids and risk of breast cancer over 20 y
of follow-up. Am J Clin Nutr. 2015;101: 1197-1205.
129. Wang Y, Gapstur SM, Gaudet MM, Furtado JD, Campos H,
McCullough ML. Plasma carotenoids and breast cancer risk in the
Cancer Prevention Study II Nutrition Cohort. Cancer Causes Control.
2015.
130. Jayasekara H, MacInnis RJ, Hodge AM, et al. Is breast cancer
risk associated with alcohol intake before first full-term pregnancy?
Cancer Causes Control. 2016;27: 1167-1174.
131. Singletary KW, Gapstur SM. Alcohol and breast cancer: review of
epidemiologic and experimental evidence and potential mechanisms.
JAMA. 2001;286: 2143-2151.
132. Jung S, Wang M, Anderson K, et al. Alcohol consumption and
breast cancer risk by estrogen receptor status: in a pooled analysis of
20 studies. Int J Epidemiol. 2016;45: 916-928.
133. Macacu A, Autier P, Boniol M, Boyle P. Active and passive
smoking and risk of breast cancer: a meta-analysis. Breast Cancer Res
Treat. 2015;154: 213-224.
134. Dossus L, Boutron-Ruault MC, Kaaks R, et al. Active and passive
cigarette smoking and breast cancer risk: results from the EPIC
cohort. Int J Cancer. 2014;134: 1871-1888.
135. Gaudet MM, Gapstur SM, Sun J, Diver WR, Hannan LM, Thun
MJ. Active smoking and breast cancer risk: original cohort data and
meta-analysis. J Natl Cancer Inst. 2013;105: 515-525.
136. White AJ, D’Aloisio AA, Nichols HB, DeRoo LA, Sandler DP.
Breast cancer and exposure to tobacco smoke during potential
windows of susceptibility. Cancer Causes Control. 2017;28: 667-675.
137. US Department of Health and Human Services. The Health
Consequences of Smoking – 50 Years of progress. A Report of the
Surgeon General. Atlanta, GA: US Department of Health and Human
Services, Centers for Disease Control and Prevention, National Center
for Chronic Disease Prevention and Health Promotion, Office on
Smoking and Health, 2014. Printed with corrections, January 2014.
138. Preston DL, Mattsson A, Holmberg E, Shore R, Hildreth NG,
Boice JD, Jr. Radiation effects on breast cancer risk: a pooled analysis
of eight cohorts. Radiat Res. 2002;158: 220-235.
139. Travis LB, Hill DA, Dores GM, et al. Breast cancer following
radiotherapy and chemotherapy among young women with Hodgkin
disease. JAMA. 2003;290: 465-475.
140. Russo J, Hu YF, Yang X, Russo IH. Developmental, cellular, and
molecular basis of human breast cancer. J Natl Cancer Inst Monogr.
2000: 17-37.
141. Schaapveld M, Aleman BM, van Eggermond AM, et al. Second
Cancer Risk Up to 40 Years after Treatment for Hodgkin’s Lymphoma.
NEJM. 2015;373: 2499-2511.
142. Moskowitz CS, Chou JF, Wolden SL, et al. Breast cancer after
chest radiation therapy for childhood cancer. J Clin Oncol. 2014;32:
2217-2223.
143. Titus-Ernstoff L, Hatch EE, Hoover RN, et al. Long-term cancer
risk in women given diethylstilbestrol (DES) during pregnancy. Br J
Cancer. 2001;84: 126-133.
144. Hoover RN, Hyer M, Pfeiffer RM, et al. Adverse health outcomes
in women exposed in utero to diethylstilbestrol. N Engl J Med.
2011;365: 1304-1314.
145. Ingber SZ, Buser MC, Pohl HR, Abadin HG, Murray HE,
Scinicariello F. DDT/DDE and breast cancer: a meta-analysis. Regul
Toxicol Pharmacol. 2013;67: 421-433.
146. Salehi F, Turner MC, Phillips KP, Wigle DT, Krewski D, Aronson
KJ. Review of the etiology of breast cancer with special attention to
organochlorines as potential endocrine disruptors. J Toxicol Environ
Health B Crit Rev. 2008;11: 276-300.
147. Calle EE, Frumkin H, Henley SJ, Savitz DA, Thun MJ.
Organochlorine and breast cancer risk. CA Cancer J Clin. 2002;52:
301-309.
148. Lopez-Cervantes M, Torres-Sanchez L, Tobias A, Lopez-Carrillo
L. Dichlorodiphenyldichloroethane burden and breast cancer risk: a
meta-analysis of the epidemiologic evidence. Environ Health Perspect.
2004;112: 207-214.
149. Cohn BA, La Merrill M, Krigbaum NY, et al. DDT Exposure in
Utero and Breast Cancer. J Clin Endocrinol Metab. 2015;100: 2865-2872.
150. Brody JG, Moysich KB, Humblet O, Attfield KR, Beehler GP, Rudel
RA. Environmental pollutants and breast cancer. Cancer. 2007;109:
2667-2711.
151. Jia Y, Lu Y, Wu K, et al. Does night work increase the risk of breast
cancer? A systematic review and meta-analysis of epidemiological
studies. Cancer Epidemiol. 2013;37: 197-206.
152. Hansen J. Night Shift Work and Risk of Breast Cancer. Curr
Environ Health Rep. 2017.
153. Wegrzyn LR, Tamimi RM, Rosner BA, et al. Rotating night shift
work and risk of breast cancer in the Nurses’ Health Studies. Amer J
Epidemiol. 2017.
154. Stevens RG, Brainard GC, Blask DE, Lockley SW, Motta ME.
Breast cancer and circadian disruption from electric lighting in the
modern world. CA Cancer J Clin. 2014;64: 207-218.
155. International Agency for Research on Cancer. IARC monographs
on the evaluation of carcinogenic risks to humans. Volume 98. Shift-
work, painting and fire-fighting. Lyon, France: International Agency
for Research on Cancer, 2007.
156. ACOG Committee Opinion No. 434: induced abortion and breast
cancer risk. Obstet Gynecol. 2009;113: 1417-1418.
157. Couzin J. Cancer risk. Review rules out abortion-cancer link.
Science. 2003;299: 1498.
158. Chen L, Malone KE, Li CI. Bra wearing not associated with breast
cancer risk: a population-based case-control study. Cancer Epidemiol
Biomarkers Prev. 2014;23: 2181-2185.
38 Breast Cancer Facts & Figures 2017-2018
159. Clemens MW, Miranda RN. Coming of Age: Breast Implant-
Associated Anaplastic Large Cell Lymphoma After 18 Years of
Investigation. Clin Plastic Surg. 2015;42: 605-613.
160. Llanos AAM, Rabkin A, Bandera EV, et al. Hair product use
and breast cancer risk among African American and White women.
Carcinogenesis. 2017.
161. Takkouche B, Etminan M, Montes-Martinez A. Personal use of
hair dyes and risk of cancer: a meta-analysis. JAMA. 2005;293: 2516-
2525.
162. Rosenberg L, Boggs DA, Adams-Campbell LL, Palmer JR. Hair
relaxers not associated with breast cancer risk: evidence from the
black women’s health study. Cancer Epidemiol Biomarkers Prev.
2007;16: 1035-1037.
163. Mirick DK, Davis S, Thomas DB. Antiperspirant use and the risk
of breast cancer. J Natl Cancer Inst. 2002;94: 1578-1580.
164. Gikas PD, Mansfield L, Mokbel K. Do underarm cosmetics cause
breast cancer? Int J Fertil Womens Med. 2004;49: 212-214.
165. Cuzick J, Sestak I, Bonanni B, et al. Selective oestrogen receptor
modulators in prevention of breast cancer: an updated meta-analysis
of individual participant data. Lancet. 2013;381: 1827-1834.
166. Goss PE, Ingle JN, Ales-Martinez JE, et al. Exemestane for breast-
cancer prevention in postmenopausal women. N Engl J Med. 2011;364:
2381-2391.
167. Cuzick J, Sestak I, Forbes JF, et al. Anastrozole for prevention
of breast cancer in high-risk postmenopausal women (IBIS-II): an
international, double-blind, randomised placebo-controlled trial.
Lancet. 2014;383: 1041-1048.
168. Ludwig KK, Neuner J, Butler A, Geurts JL, Kong AL. Risk
reduction and survival benefit of prophylactic surgery in BRCA
mutation carriers, a systematic review. Am J Surg. 2016;212: 660-669.
169. Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk
reduction estimates associated with risk-reducing salpingo-
oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer
Inst. 2009;101: 80-87.
170. Domchek SM, Friebel TM, Singer CF, et al. Association of risk-
reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer
risk and mortality. JAMA. 2010;304: 967-975.
171. Kotsopoulos J, Huzarski T, Gronwald J, et al. Bilateral
Oophorectomy and Breast Cancer Risk in BRCA1 and BRCA2
Mutation Carriers. J Natl Cancer Inst. 2017;109.
172. Oeffinger KC, Fontham ET, Etzioni R, et al. Breast Cancer
Screening for Women at Average Risk: 2015 Guideline Update From
the American Cancer Society. JAMA. 2015;314: 1599-1614.
173. Pisano ED, Hendrick RE, Yaffe MJ, et al. Diagnostic accuracy of
digital versus film mammography: exploratory analysis of selected
population subgroups in DMIST. Radiology. 2008;246: 376-383.
174. Kerlikowske K, Hubbard RA, Miglioretti DL, et al. Comparative
effectiveness of digital versus film-screen mammography in
community practice in the United States: a cohort study. Ann Intern
Med. 2011;155: 493-502.
175. Souza FH, Wendland EM, Rosa MI, Polanczyk CA. Is full-
field digital mammography more accurate than screen-film
mammography in overall population screening? A systematic review
and meta-analysis. Breast. 2013.
176. Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer
screening using tomosynthesis in combination with digital
mammography. JAMA. 2014;311: 2499-2507.
177. Bernardi D, Macaskill P, Pellegrini M, et al. Breast cancer
screening with tomosynthesis (3D mammography) with acquired
or synthetic 2D mammography compared with 2D mammography
alone (STORM-2): a population-based prospective study. Lancet
Oncol. 2016;17: 1105-1113.
178. Conant EF, Beaber EF, Sprague BL, et al. Breast cancer screening
using tomosynthesis in combination with digital mammography
compared to digital mammography alone: a cohort study within the
PROSPR consortium. Breast Cancer Res Treat. 2016;156: 109-116.
179. Independent U. K. Panel on Breast Cancer Screening. The
benefits and harms of breast cancer screening: an independent
review. Lancet. 2012;380: 1778-1786.
180. Coldman A, Phillips N, Wilson C, et al. Pan-Canadian study of
mammography screening and mortality from breast cancer. J Natl
Cancer Inst. 2014;106.
181. Hofvind S, Ursin G, Tretli S, Sebuodegard S, Moller B. Breast
cancer mortality in participants of the Norwegian Breast Cancer
Screening Program. Cancer. 2013;119: 3106-3112.
182. Hubbard RA, Kerlikowske K, Flowers CI, Yankaskas BC, Zhu
W, Miglioretti DL. Cumulative probability of false-positive recall or
biopsy recommendation after 10 years of screening mammography: a
cohort study. Ann Intern Med. 2011;155: 481-492.
183. Kerlikowske K, Zhu W, Hubbard RA, et al. Outcomes of screening
mammography by frequency, breast density, and postmenopausal
hormone therapy. JAMA Intern Med. 2013;173: 807-816.
184. Lehman CD, Arao RF, Sprague BL, et al. National Performance
Benchmarks for Modern Screening Digital Mammography: Update
from the Breast Cancer Surveillance Consortium. Radiology. 2017;283:
49-58.
185. Elmore JG, Barton MB, Moceri VM, Polk S, Arena PJ, Fletcher SW.
Ten-year risk of false positive screening mammograms and clinical
breast examinations. N Engl J Med. 1998;338: 1089-1096.
186. Gotzsche PC, Jorgensen KJ. Screening for breast cancer with
mammography. Cochrane Database Syst Rev. 2013;6: CD001877.
187. Marmot MG, Altman DG, Cameron DA, Dewar JA, Thompson
SG, Wilcox M. The benefits and harms of breast cancer screening: an
independent review. Br J Cancer. 2013;108: 2205-2240.
188. Nelson HD, Tyne K, Naik A, et al. Screening for Breast Cancer:
Systematic Evidence Review Update for the US Preventive Services Task
Force. Rockville (MD), 2009.
189. Duffy SW, Tabar L, Olsen AH, et al. Absolute numbers of
lives saved and overdiagnosis in breast cancer screening, from a
randomized trial and from the Breast Screening Programme in
England. J Med Screen. 2010;17: 25-30.
190. Puliti D, Zappa M, Miccinesi G, Falini P, Crocetti E, Paci E. An
estimate of overdiagnosis 15 years after the start of mammographic
screening in Florence. Eur J Cancer. 2009;45: 3166-3171.
191. Lauby-Secretan B, Scoccianti C, Loomis D, et al. Breast-cancer
screening – viewpoint of the IARC Working Group. N Engl J Med.
2015;372: 2353-2358.
192. Yaffe MJ, Mainprize JG. Risk of radiation-induced breast cancer
from mammographic screening. Radiology. 2011;258: 98-105.
193. de Gelder R, Draisma G, Heijnsdijk EA, de Koning HJ. Population-
based mammography screening below age 50: balancing radiation-
induced vs prevented breast cancer deaths. Br J Cancer. 2011;104:
1214-1220.
Breast Cancer Facts & Figures 2017-2018 39
194. Brawley OW. Risk-based mammography screening: an effort to
maximize the benefits and minimize the harms. Ann Intern Med.
2012;156: 662-663.
195. National Center for Health Statistics. National Health Interview
Survey, 2015. Public-use data file and documentation.
http://www.cdc.
gov/nchs/nhis.htm
.
196. Centers for Disease Control and Prevention (CDC). Behavioral
Risk Factor Surveillance System Survey Data. Atlanta, Georgia: U.S.
Department of Health and Human Services, Centers for Disease
Control and Prevention, 2017.
197. Lantz PM, Mullen J. The National Breast and Cervical Cancer
Early Detection Program: 25 Years of public health service to low-
income women. Cancer Causes Control. 2015;26: 653-656.
198. Centers for Disease Control and Prevention. National Breast
and Cervical Cancer Early Detection Program (NBCCEDP). Available
from URL:
http://www.cdc.gov/cancer/nbccedp/about.htm
Accessed
05/20/2015.
199. Saslow D, Boetes C, Burke W, et al. American Cancer Society
guidelines for breast screening with MRI as an adjunct to
mammography. CA Cancer J Clin. 2007;57: 75-89.
200. Haas JS, Hill DA, Wellman RD, et al. Disparities in the use of
screening magnetic resonance imaging of the breast in community
practice by race, ethnicity, and socioeconomic status. Cancer.
2016;122: 611-617.
201. Tagliafico AS, Calabrese M, Mariscotti G, et al. Adjunct
Screening With Tomosynthesis or Ultrasound in Women With
Mammography-Negative Dense Breasts: Interim Report of a
Prospective Comparative Trial. J Clin Oncol. 2016.
202. Bobo JK, Lee NC, Thames SF. Findings from 752,081 clinical
breast examinations reported to a national screening program from
1995 through 1998. J Natl Cancer Inst. 2000;92: 971-976.
203. McDonald S, Saslow D, Alciati MH. Performance and reporting
of clinical breast examination: a review of the literature. CA Cancer J
Clin. 2004;54: 345-361.
204. Smith RA, Saslow D, Sawyer KA, et al. American Cancer Society
guidelines for breast cancer screening: update 2003. CA Cancer J Clin.
2003;53: 141-169.
205. Thomas DB, Gao DL, Ray RM, et al. Randomized trial of breast
self-examination in Shanghai: final results. J Natl Cancer Inst.
2002;94: 1445-1457.
206. Semiglazov VF, Moiseenko VM, Manikhas AG, et al. Interim
results of a prospective randomized study of self-examination for
early detection of breast cancer. Vopr Onkol. 1999;45: 265-271.
207. Francis A, Thomas J, Fallowfield L, et al. Addressing
overtreatment of screen detected DCIS; the LORIS trial. Eur J Cancer.
2015;51: 2296-2303.
208. Elshof LE, Tryfonidis K, Slaets L, et al. Feasibility of a
prospective, randomised, open-label, international multicentre,
phase III, non-inferiority trial to assess the safety of active
surveillance for low risk ductal carcinoma in situ – The LORD study.
Eur J Cancer. 2015;51: 1497-1510.
209. ClinicalTrials.gov. Comparison of Operative to Monitoring and
Endocrine Therapy (COMET) Trial For Low Risk DCIS: A Phase III
Prospective Randomized Trial. Available from URL:
https://clinicaltrials.
gov/ct2/show/study/NCT02926911
Accessed May 16, 2017.
210. Benson JR, Jatoi I, Toi M. Treatment of low-risk ductal carcinoma
in situ: is nothing better than something? Lancet Oncol. 2016;17:
e442-e451.
211. Wazir U, Wazir A, Wells C, Mokbel K. Pleomorphic lobular
carcinoma in situ: Current evidence and a systemic review. Oncol
Lett. 2016;12: 4863-4868.
212. Chen Y, Jiang L, Gao B, Cheng ZY, Jin J, Yang KH. Survival and
disease-free benefits with mastectomy versus breast conservation
therapy for early breast cancer: a meta-analysis. Breast Cancer Res
Treat. 2016;157: 517-525.
213. Early Breast Cancer Trialists’ Collaborative Group, Darby
S, McGale P, et al. Effect of radiotherapy after breast-conserving
surgery on 10-year recurrence and 15-year breast cancer death:
meta-analysis of individual patient data for 10,801 women in 17
randomised trials. Lancet. 2011;378: 1707-1716.
214. van Maaren MC, de Munck L, de Bock GH, et al. 10 year survival
after breast-conserving surgery plus radiotherapy compared with
mastectomy in early breast cancer in the Netherlands: a population-
based study. Lancet Oncol. 2016.
215. Hartmann-Johnsen OJ, Karesen R, Schlichting E, Nygard JF.
Survival is Better After Breast Conserving Therapy than Mastectomy
for Early Stage Breast Cancer: A Registry-Based Follow-up Study of
Norwegian Women Primary Operated Between 1998 and 2008. Ann
Surg Oncol. 2015;22: 3836-3845.
216. Hwang ES, Lichtensztajn DY, Gomez SL, Fowble B, Clarke CA.
Survival after lumpectomy and mastectomy for early stage invasive
breast cancer: the effect of age and hormone receptor status. Cancer.
2013;119: 1402-1411.
217. Agarwal S, Pappas L, Neumayer L, Kokeny K, Agarwal J. Effect
of breast conservation therapy vs mastectomy on disease-specific
survival for early-stage breast cancer. JAMA Surg. 2014;149: 267-274.
218. Smith BD, Jiang J, Shih YC, et al. Cost and Complications of Local
Therapies for Early-Stage Breast Cancer. J Natl Cancer Inst. 2017;109.
219. Lautner M, Lin H, Shen Y, et al. Disparities in the Use of Breast-
Conserving Therapy Among Patients With Early-Stage Breast Cancer.
JAMA Surg. 2015;150: 778-786.
220. Kummerow KL, Du L, Penson DF, Shyr Y, Hooks MA. Nationwide
trends in mastectomy for early-stage breast cancer. JAMA surgery.
2015;150: 9-16.
221. McGuire KP, Santillan AA, Kaur P, et al. Are mastectomies on
the rise? A 13-year trend analysis of the selection of mastectomy
versus breast conservation therapy in 5865 patients. Ann Surg Oncol.
2009;16: 2682-2690.
222. Bellavance EC, Kesmodel SB. Decision-Making in the Surgical
Treatment of Breast Cancer: Factors Influencing Women’s Choices for
Mastectomy and Breast Conserving Surgery. Front Oncol. 2016;6: 74.
223. Freedman RA, Virgo KS, Labadie J, He Y, Partridge AH, Keating
NL. Receipt of locoregional therapy among young women with breast
cancer. Breast Cancer Res Treat. 2012;135: 893-906.
224. Wong SM, Freedman RA, Sagara Y, Aydogan F, Barry WT,
Golshan M. Growing Use of Contralateral Prophylactic Mastectomy
Despite no Improvement in Long-term Survival for Invasive Breast
Cancer. Ann Surg. 2016.
225. Kurian AW, Lichtensztajn DY, Keegan TH, Nelson DO, Clarke CA,
Gomez SL. Use of and mortality after bilateral mastectomy compared
with other surgical treatments for breast cancer in California, 1998-
2011. JAMA. 2014;312: 902-914.
226. Tuttle TM, Jarosek S, Habermann EB, et al. Increasing rates of
contralateral prophylactic mastectomy among patients with ductal
carcinoma in situ. J Clin Oncol. 2009;27: 1362-1367.
40 Breast Cancer Facts & Figures 2017-2018
227. Fayanju OM, Stoll CR, Fowler S, Colditz GA, Margenthaler JA.
Contralateral prophylactic mastectomy after unilateral breast
cancer: a systematic review and meta-analysis. Ann Surg. 2014;260:
1000-1010.
228. Boughey JC, Attai DJ, Chen SL, et al. Contralateral Prophylactic
Mastectomy (CPM) Consensus Statement from the American Society
of Breast Surgeons: Data on CPM Outcomes and Risks. Ann Surg
Oncol. 2016;23: 3100-3105.
229. Lyman GH, Somerfield MR, Bosserman LD, Perkins CL, Weaver
DL, Giuliano AE. Sentinel Lymph Node Biopsy for Patients With
Early-Stage Breast Cancer: American Society of Clinical Oncology
Clinical Practice Guideline Update. J Clin Oncol. 2016: JCO2016710947.
230. Giuliano AE, Ballman K, McCall L, et al. Locoregional
Recurrence After Sentinel Lymph Node Dissection With or
Without Axillary Dissection in Patients With Sentinel Lymph Node
Metastases: Long-term Follow-up From the American College of
Surgeons Oncology Group (Alliance) ACOSOG Z0011 Randomized
Trial. Ann Surg. 2016;264: 413-420.
231. DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm
lymphoedema after breast cancer: a systematic review and meta-
analysis. Lancet Oncol. 2013;14: 500-515.
232. Cheema BS, Kilbreath SL, Fahey PP, Delaney GP, Atlantis E.
Safety and efficacy of progressive resistance training in breast
cancer: a systematic review and meta-analysis. Breast Cancer Res
Treat. 2014;148: 249-268.
233. Lawenda BD, Mondry TE, Johnstone PA. Lymphedema: a primer
on the identification and management of a chronic condition in
oncologic treatment. CA Cancer J Clin. 2009;59: 8-24.
234. Hughes KS, Schnaper LA, Berry D, et al. Lumpectomy plus
tamoxifen with or without irradiation in women 70 years of age or
older with early breast cancer. N Engl J Med. 2004;351: 971-977.
235. Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation
of Breast Radiotherapy (START) trials of radiotherapy
hypofractionation for treatment of early breast cancer: 10-year
follow-up results of two randomised controlled trials. Lancet Oncol.
2013;14: 1086-1094.
236. Shaitelman SF, Schlembach PJ, Arzu I, et al. Acute and
Short-term Toxic Effects of Conventionally Fractionated vs
Hypofractionated Whole-Breast Irradiation: A Randomized Clinical
Trial. JAMA Oncol. 2015;1: 931-941.
237. Shah C, Badiyan S, Ben Wilkinson J, et al. Treatment efficacy
with accelerated partial breast irradiation (APBI): final analysis of
the American Society of Breast Surgeons MammoSite((R)) breast
brachytherapy registry trial. Ann Surg Oncol. 2013;20: 3279-3285.
238. Strnad V, Ott OJ, Hildebrandt G, et al. 5-year results of
accelerated partial breast irradiation using sole interstitial
multicatheter brachytherapy versus whole-breast irradiation with
boost after breast-conserving surgery for low-risk invasive and
in-situ carcinoma of the female breast: a randomised, phase 3, non-
inferiority trial. Lancet. 2016;387: 229-238.
239. Krug D, Baumann R, Budach W, et al. Current controversies in
radiotherapy for breast cancer. Radiat Oncol. 2017;12: 25.
240. Shah C, Tendulkar R, Smile T, et al. Adjuvant Radiotherapy in
Early-Stage Breast Cancer: Evidence-Based Options. Ann Surg Oncol.
2016;23: 3880-3890.
241. Zhang L, Zhou Z, Mei X, et al. Intraoperative Radiotherapy
Versus Whole-Breast External Beam Radiotherapy in Early-Stage
Breast Cancer: A Systematic Review and Meta-Analysis. Medicine.
2015;94: e1143.
242. Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant
systemic treatment in breast cancer: a meta-analysis. J Natl Cancer
Inst. 2005;97: 188-194.
243. Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox:
primary tumor chemosensitivity of breast cancer subtypes. Clin
Cancer Res. 2007;13: 2329-2334.
244. Sparano JA, Gray RJ, Makower DF, et al. Prospective Validation of
a 21-Gene Expression Assay in Breast Cancer. N Engl J Med. 2015;373:
2005-2014.
245. Early Breast Cancer Trialists’ Collaborative Group, Davies C,
Godwin J, et al. Relevance of breast cancer hormone receptors and
other factors to the efficacy of adjuvant tamoxifen: patient-level
meta-analysis of randomised trials. Lancet. 2011;378: 771-784.
246. Davies C, Pan H, Godwin J, et al. Long-term effects of continuing
adjuvant tamoxifen to 10 years versus stopping at 5 years after
diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a
randomised trial. Lancet. 2013;381: 805-816.
247. Burstein HJ, Temin S, Anderson H, et al. Adjuvant endocrine
therapy for women with hormone receptor-positive breast cancer:
American Society of Clinical Oncology clinical practice guideline
focused update. J Clin Oncol. 2014;32: 2255-2269.
248. Dowsett M, Cuzick J, Ingle J, et al. Meta-analysis of breast
cancer outcomes in adjuvant trials of aromatase inhibitors versus
tamoxifen. J Clin Oncol. 2010;28: 509-518.
249. Pagani O, Regan MM, Walley BA, et al. Adjuvant exemestane
with ovarian suppression in premenopausal breast cancer. N Engl J
Med. 2014;371: 107-118.
250. Klijn JG, Blamey RW, Boccardo F, et al. Combined tamoxifen
and luteinizing hormone-releasing hormone (LHRH) agonist versus
LHRH agonist alone in premenopausal advanced breast cancer: a
meta-analysis of four randomized trials. J Clin Oncol. 2001;19: 343-353.
251. Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus
adjuvant chemotherapy for operable HER2-positive breast cancer. N
Engl J Med. 2005;353: 1673-1684.
252. Runowicz CD, Leach CR, Henry NL, et al. American Cancer
Society/American Society of Clinical Oncology Breast Cancer
Survivorship Care Guideline. CA Cancer J Clin. 2016;66: 43-73.
253. Copeland G, Lake A, Firth R, et al. Cancer in North America: 2010-
2014. Volume Two: Registry-specific Cancer Incidence for the United
States, Canada and North America. Springfield, IL: North American
Association of Central Cancer Registries, Inc, June 2017.
254. Copeland G, Lake A, Firth R, et al. Cancer in North America:
2010-2014. Volume One: Combined Cancer Incidence for the United
States, Canada and North America. Springfield, IL: North American
Association of Central Cancer Registries, Inc, June 2017.
255. Surveillance, Epidemiology, and End Results (SEER) Program
(
www.seer.cancer.gov
) SEER*Stat Database: Incidence – SEER 9 Regs
Research Data, Nov 2016 Sub (1973-2014) <Katrina/Rita Population
Adjustment> Total U.S., 1969-2015 Counties, National Cancer
Institute, DCCPS, Surveillance Research Program, released April
2017, based on the November 2016 submission.
256. Surveillance Research Program, National Cancer Institute.
SEER*Stat Software. Version 8.3.4. Bethesda, MD: National Cancer
Institute; 2017.
257. DevCan: Probability of Developing or Dying of Cancer Software,
Version 6.7.5; Statistical Research and Applications Branch, National
Cancer Institute, 2017.
https://surveillance.cancer.gov/devcan/
.

Breast Cancer Facts & Figures 2017-2018 41
Acknowledgments
The production of this report would not have been possible without the efforts of:
Rick Alteri, MD; Ted Gansler, MD, MPH; Mia M Gaudet, PhD; Gretchen Gierach, PhD, MPH; Mamta Kalidas, MD; Joan
Kramer, MD; Katie McMahon, MPH; Kimberly Miller, MPH; Lisa A Newman, MD, MPH; Anthony Piercy; Cheri Richards, MS;
Ann Goding Sauer, MSPH; Scott Simpson; Robert Smith, PhD; Lindsey Torre, MSPH; Dana Wagner; and Jiaquan Xu, MD.
Breast Cancer Facts & Figures is a biennial publication of the American Cancer Society, Atlanta, Georgia.
For more information, contact:
Carol DeSantis, MPH; Rebecca Siegel, MPH; Ahmedin Jemal, DVM, PhD
Surveillance and Health Services Research Program

©2017, American Cancer Society, Inc.
No. 861017
Models used for illustrative purposes only.
The American Cancer Society’s mission
is to save lives, celebrate lives,
and lead the fight for a world without cancer.
