Chapter 21 Review Questions and Text Homework Solutions
Review Questions
1. A hydrocarbon is a compound composed of only carbon and hydrogen. A saturated hydro-carbon
has only carbon-carbon single bonds in the molecule. An unsaturated hydrocarbon has one or more
carbon-carbon multiple bonds but may also contain carbon-carbon single bonds. A normal
hydrocarbon has one chain of consecutively bonded carbon atoms, with each carbon atom in the
chain bonded to one or two other carbon atoms. A branched hydrocarbon has at least one carbon
atom in the structure that forms bonds to three or four other carbon atoms; the structure is not one
continuous chain of carbon atoms.
An alkane is a saturated hydrocarbon composed of only C−C and C−H single bonds. Each carbon
in an alkane is bonded to four other atoms (either C or H atoms). If the compound contains a ring
in the structure and is composed of only C−C and C−H single bonds, then it is called a cyclic alkane.
Alkanes: general formula = C
n
H
2n + 2
; all carbons are sp
3
hybridized; bond angles = 109.5˚
Cyclic alkanes: general formula C
n
H
2n
(if only one ring is present in the compound); all carbons
are sp
3
hybridized; prefers 109.5˚ bond angles, but rings with three carbons or four carbons or five
carbons are forced into bond angles less than 109.5˚.
In cyclopropane, a ring compound made up of three carbon atoms, the bond angles are forced into
60˚ in order to form the three-carbon ring. With four bonds to each carbon, the carbons prefer
109.5˚ bond angles. This just can’t happen for cyclopropane. Because cyclopropane is forced to
form bond angles smaller than it prefers, it is very reactive.
The same is true for cyclobutane. Cyclobutane is composed of a four-carbon ring. In order to form
a ring compound with four carbons, the carbons in the ring are forced to form 90˚ bond angles; this
is much smaller than the preferred 109.5˚ bond angles.
Cyclopentane (five-carbon rings) also has bond angles slightly smaller than 109.5˚, but they are
very close (108˚), so cyclopentane is much more stable than cyclopropane or cyclobutane. For rings
having six or more carbons, the observed bonds are all 109.5˚.
Straight chain hydrocarbons just indicates that there is one chain of consecutively bonded C-atoms
in the molecule. They are not in a straight line which infers 180˚ bond angles. The bond angles are
the predicted 109.5˚.
To determine the number of hydrogens bonded to the carbons in cyclic alkanes (or any alkane
where they may have been omitted), just remember that each carbon has four bonds. In
cycloalkanes, only the C−C bonds are shown. It is assumed you know that the remaining bonds on
each carbon are C−H bonds. The number of C−H bonds is that number required to give the carbon
four total bonds.

2. Alkenes are unsaturated hydrocarbons that contain a carbon-carbon double bond. Carbon-carbon
single bonds may also be present. Alkynes are unsaturated hydrocarbons that contain a carbon-
carbon triple bond.
Alkenes: C
n
H
2n
is the general formula. The carbon atoms in the C=C bond exhibit 120˚ bond
angles. The double-bonded carbon atoms are sp
2
hybridized. The three sp
2
hybrid orbitals form
three sigma bonds to the attached atoms. The unhybridized p atomic orbital on each sp
2
hybridized
carbon overlap side to side to form the bond in the double bond. Because the p orbitals must
overlap parallel to each other, there is no rotation in the double bond (this is true whenever bonds
are present). See Figure 21.7 for the bonding in the simplest alkene, C
2
H
4
.
Alkynes: C
n
H
2n – 2
is the general formula. The carbon atoms in the C−C bond exhibit 180˚ bond
angles. The triple bonded carbons are sp hybridized. The two sp hybrid orbitals form two sigma
bonds to the bonded atoms. The two unhybridized p atomic orbitals overlap with two unhybridized
p atomic orbitals on the other carbon in the triple bond, forming two bonds. If the z-axis is the
internuclear axis, then one bond would form by parallel overlap of p
y
orbitals on each carbon and
the other bond would form by parallel overlap of p
x
orbitals. As is the case with alkenes, alkynes
have restricted rotation due to the bonds. See Figure 21.10 for the bonding in the simplest alkyne,
C
2
H
2
.
Any time a multiple bond or a ring structure is added to a hydrocarbon, two hydrogens are lost from
the general formula. The general formula for a hydrocarbon having one double bond and one ring
structure would lose four hydrogens from the alkane general formula. The general formula would
be C
n
H
2n – 2
.
3. Aromatic hydrocarbons are a special class of unsaturated hydrocarbons based on the benzene ring.
Benzene has the formula C
6
H
6
. It is a planar molecule (all atoms are in the same plane). The
bonding in benzene is discussed in detail in Section 4.7 of the text. Figures 4.57-4.59 detail the
bonding in benzene.
C
6
H
6
, has 6(4) + 6(1) = 30 valence electrons. The two resonance Lewis structures for benzene are:
These are abbreviated as:

Each carbon in benzene is attached to three other atoms; it exhibits trigonal planar geometry with
120° bond angles. Each carbon is sp
2
hybridized. The sp
2
hybrid orbitals form three sigma bonds
to each carbon. The unhybridized p atomic orbital on each carbon overlap side to side with
unhybridized p orbitals on adjacent carbons to form the bonds. All six of the carbons in the six-
membered ring have one unhybridized p atomic orbital. All six of the unhybridized p orbitals
overlap side to side to give a ring of electron density above and below the six-membered ring of
benzene.
The six electrons in the bonds in benzene can roam about above and below the entire ring
surface; these electrons are delocalized. This is important because all six carbon-carbon bonds in
benzene are equivalent in length and strength. The Lewis structures say something different (three
of the bonds are single and three of the bonds are double). This is not correct. To explain the
equivalent bonds, the bonds can’t be situated between two carbon atoms as is the case in alkenes
and alkynes; that is, the bonds can’t be localized. Instead, the six electrons can roam about over
a much larger area; they are delocalized over the entire surface of the molecule. All this is implied
in the following shorthand notation for benzene.
4. A short summary of the nomenclature rules for alkanes, alkenes, and alkynes follow. See the text
for details.
a. Memorize the base names of C
1
–C
10
carbon chains (see Table 21.1). When the C
1
–C
10
carbon chains are named as a substituent, change the –ane suffix to–yl.
b. Memorize the additional substituent groups in Table 21.2.
c. Names are based on the longest continuous carbon chain in the molecule. Alkanes use the
suffix –ane, alkenes end in –ene, and alkynes end in –yne.
d. To indicate the position of a branch or substituent, number the longest chain of carbons
consecutively in order to give the lowest numbers to the substituents or branches. Identify the
number of the carbon that the substituent is bonded to by writing the number in front of the
name of the substituent.
e. Name substituents in alphabetical order.
f. Use a prefix (di–, tri–, tetra–, etc.) to indicate the number of a substituent if more than one is
present. Note that if, for example, three methyl substituent groups are bonded to carbons on the
longest chain, use the tri-prefix but also include three numbers indicating the positions of the
methyl groups on the longest chain. Also note that prefixes like di–, tri–, tetra–, etc. are not
considered when determining the alphabetical order of the substituent groups.
g. A cyclic hydrocarbon is designated by the prefix cyclo–.
h. For alkenes and alkynes, the position of the double or triple bond is indicated with a number
placed directly in front of the base name of the longest chain. If more than one multiple bond
is present, the number of multiple bonds is indicted in the base name using the prefix di–, tri–,
tetra–, etc., but also a number for the position of each multiple bond is indicated in front of the
base name. When numbering the longest chain, if double or triple bonds are present, give the
multiple bonds the lowest number possible (not the substituent groups).
This is a start. As you will find out, there are many interesting situations that can come up which
aren’t covered by these rules. We will discuss them as they come up.
For aromatic nomenclature rules, reference Section 21.3 of the text.
The errors in the names are discussed below.
a. The longest chain gives the base name.
b. The suffix –ane indicates only alkanes. Alkenes and alkynes have different suffixes as do other
“types” of organic compounds.
c. Smallest numbers are used to indicate the position of substituents.
d. Numbers are required to indicate the positions of double or triple bonds.
e. Multiple bonds (double or triple) get the lowest number.
f. The term ortho– in benzene nomenclature indicates substituents in the benzene ring bonded to
C–1 and C–2. The term meta– describes C–1 and C–3 substituent groups, while para– is used
for C–1 and C–4 substituent groups.
6. Resonance: All atoms are in the same position. Only the positions of electrons are
different.
Isomerism: Atoms are in different locations in space.
Isomers are distinctly different substances. Resonance is the use of more than one Lewis structure
to describe the bonding in a single compound. Resonance structures are n ot isomers.
Structural isomers: Same formula but different bonding, either in the kinds of bonds present or the
way in which the bonds connect atoms to each other.
Geometrical isomers: Same formula and same bonds, but differ in the arrangement of atoms in
space about a rigid bond or ring.
To distinguish isomers from molecules that differ by rotations about some bonds, name them. If
two structures have different names, they are different isomers (different compounds). If the two
structures have the same name, then they are the same compound. The two compounds may look
different, but if they have the same names, they are the same compounds that only differ by some
rotations about single bonds in the molecule.
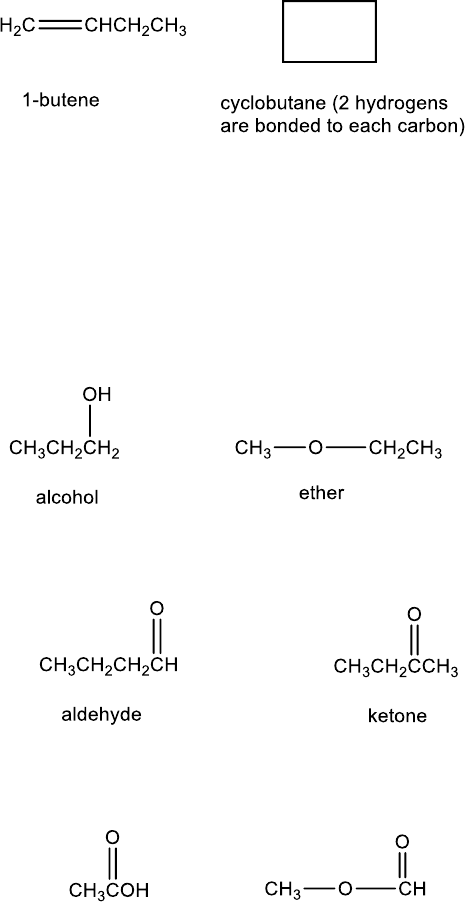
An alkene and a cyclic alkane having the C
4
H
8
formula are:
For cis-trans isomerism (geometric isomerism), you must have at least two carbons with restricted
rotation (double bond or ring) that each have two different groups bonded to it. The cis isomer will
generally have the largest groups bonded to the two carbons with restricted rotation on the same
side of the double bond or ring. The trans isomer generally has the largest groups bonded to the
two carbons with restricted rotation on opposite sides of the double bond or ring.
For alcohols and ethers, consider the formula C
3
H
8
O. An alcohol and an ether that have this formula
are:
For aldehydes and ketones, consider the formula C
4
H
8
O. An aldehyde and a ketone that have this
formula are:
Esters are structural isomers of carboxylic acids. An ester and a carboxylic acid having the formula
C
2
H
4
O
2
are:
carboxylic acid ester
Optical isomers: The same formula and the same bonds, but the compounds are nonsuper-
imposable mirror images of each other. The key to identifying optical isomerism in organic
compounds is to look for a tetrahedral carbon atom with four different substituents attached. When
four different groups are bonded to a carbon atom, then a nonsuperimposable mirror image does
exist.

1−bromo−1−chloroethane 1−bromo−2−chloroethane
The carbon with the asterisk has 4
different groups bonded to it (1−Br;
2−Cl; 3−CH
3
; 4−H). This compound
has a nonsuperimposable mirror
image.
Neither of the two carbons have four different
groups bonded to it. The mirror image of this
molecule will be superimposable (it does not
exhibit optical isomerism).
Text Homework
16. a.
CH
3
CCH
2
CH
2
CH
2
CH
3
CH
3
CH
3
CH
3
CHCHCH
2
CH
2
CH
3
CH
3
CH
3
2,2-dimethylhexane 2,3-dimethylhexane
3-ethylhexane
CH
3
CH
2
CCH
2
CH
2
CH
3
CH
3
CH
3
CH
3
CH
2
CHCHCH
2
CH
3
CH
3
CH
3
3,3-dimethylhexane
3,4-dimethylhexane
CH
3
CH
2
CHCH
2
CH
2
CH
3
CH
2
CH
3
CH
3
CHCH
2
CHCH
2
CH
3
CH
3
CH
3
CH
3
CHCH
2
CH
2
CHCH
3
CH
3
CH
3
2,4-dimethylhexane
2,5-dimethylhexane

CH
3
C CH CH
2
CH
3
CH
3
CH
3
CH
3
C CH
2
CH CH
3
CH
3
CH
3
CH
3
2,2,3-trimethylpentane 2,2,4-trimethylpentane
CH
3
CH C CH
2
CH
3
CH
3
CH
3
CH
3
CH
3
CH CH CH CH
3
CH
3
CH
3
CH
3
2,3,3-trimethylpentane 2,3,4-trimethylpentane
CH
3
CH CH CH
2
CH
3
CH
3
CH
2
CH
3
CH
3
CH
2
C CH
2
CH
3
CH
3
CH
2
CH
3
3-ethyl-2-methylpentane 3-ethyl-3-methylpentane
H
3
C
b.
34. a. All these structures have the formula C
5
H
8
. The compounds with the same physical
properties will be the compounds that are identical to each other, i.e., compounds that only
differ by rotations of C−C single bonds. To recognize identical compounds, name them. The
names of the compounds are:
i. trans-1,3-pentadiene ii. cis-1,3-pentadiene
iii. cis-1,3-pentadiene iv. 2-methyl-1,3-butadiene
Compounds ii and iii are identical compounds, so they would have the same physical
properties.
b. Compound i is a trans isomer because the bulkiest groups off the C
3
=C
4
double bond are on
opposite sides of the double bond.
c. Compound iv does not have carbon atoms in a double bond that each have two different groups
attached. Compound iv does not exhibit cis-trans isomerism.
42. The cis isomer has the CH
3
groups on the same side of the ring. The trans isomer has the CH
3
groups on opposite sides of the ring.
trans
CH
3
H
CH
3
H
cis
H
CH
3
CH
3
H

The cyclic structural and geometric isomers of C
4
H
7
F are:
46. a.
b. There are three trichlorobenzenes (1,2,3-trichlorobenzene, 1,2,4-trichlorobenzene, and
1,3,5-trichlorobenzene).
c. The meta isomer will be very difficult to synthesize.
d. 1,3,5-Trichlorobenzene will be the most difficult to synthesize since all Cl groups are
meta to each other in this compound.
62. a. The two possible products for the addition of HOH to this alkene are:
We would get both products in this reaction. Using the rule given in the problem, the
first compound listed is the major product. In the reactant, the terminal carbon has
more hydrogens bonded to it (2 versus 1), so H forms a bond to this carbon, and OH
forms a bond to the other carbon in the double bond for the major product. We will list
only the major product for the remaining parts to this problem.
b. c.
F
trans
cis
F
CH
3
F CH
3
CH
3
F
CH
2
F
CH
3
CH
2
CH CH
2
OH H
CH
3
CH
2
CH CH
2
H
OH
major product minor product
CH
3
CH
2
CH CH
2
Br H
CH
3
CH
2
C CH
Br
Br
H
H
Cl
Cl
Cl
Cl
Cl
Cl
ortho parameta

CH
3
CH
2
C C CH
3
Cl
CH
3
H
H
d. e.
64. When CH
2
=CH
2
reacts with HCl, there is only one possible product, chloroethane. When
Cl
2
is reacted with CH
3
CH
3
(in the presence of light), there are six possible products
because any number of the six hydrogens in ethane can be substituted for by Cl. The light-
catalyzed substitution reaction is very difficult to control; hence it is not a very efficient
method of producing monochlorinated alkanes.
104.
The compound has four chiral carbon atoms (marked with *). The fourth group bonded to
the three chiral carbon atoms in the ring is a hydrogen atom.
154. The five chiral carbons are marked with an asterisk.
Each of these five carbons has four different groups bonded to it. The fourth bond that is
not shown for any of the five chiral carbons is a C−H bond.
*
O
*
H
3
C C H
OH
OH
OH
*
*
CH
3
OH
*
*
*
*
*
HO
CH
3
OH
H
