
EUTHANASIA
REFERENCE
MANUAL
The Humane Society of the United States

EUTHANASIA
REFERENCE
MANUAL
The Humane Society of the United States

ii
The Humane Society of the United States Euthanasia Reference Manual
Copyright © 2013 by The Humane Society of the United States. All rights reserved.
No portion of this book may be reproduced in any form or by any electronic or mechanical means,
including information storage and retrieval systems, without permission in writing from the publisher.
Second edition
ISBN 978-1-934785-03-4
Library of Congress Cataloging-in-Publication Number: TXu 1-866-347
Copyright © 2013 by The Humane Society of the United States. All rights reserved.
No portion of this book may be reproduced in any form or by any electronic or mechanical means,
including information storage and retrieval systems, without permission in writing from the publisher.
Second edition
ISBN 978-1-934785-04-1
Library of Congress Cataloging-in-Publication Number: TXu 1-866-347

iii
The Humane Society of the United States Euthanasia Reference Manual
Table of Contents
Acknowledgments ..........................................................................vii
Foreword ..................................................................................... viii
CHAPTER 1:
Understanding Euthanasia .................................................................1
What Is Euthanasia? .........................................................................1
Euthanasia, Past and Present ................................................................1
Important Definitions .......................................................................3
CHAPTER 2:
Sodium Pentobarbital .......................................................................4
How Sodium Pentobarbital Works .........................................................4
Administering Sodium Pentobarbital .......................................................7
Intravenous (IV) Injection (Injection of Sodium Pentobarbital Directly into a Vein) .............. 7
Intraperitoneal (IP) Injection (Injection of Sodium Pentobarbital
into the Abdominal Cavity) .................................................................22
Intracardiac (IC) Injection (Injection of Sodium Pentobarbital Directly into the Heart) ..........26
Other Injection Routes—Not Acceptable .................................................... 29
Oral Administration of Sodium Pentobarbital (PO) ..........................................30
CHAPTER 3:
Pre-Euthanasia Drugs ......................................................................31
Advantages .................................................................................31
Disadvantages ..............................................................................31
Policy ........................................................................................32
Types of Pre-Euthanasia Drugs .............................................................33
Best Pre-Euthanasia Drug Option A: PreMix (Xylazine/Ketamine Combination) ..............33
Best Pre-Euthanasia Drug Option B: Telazol (Tiletamine/Zolazepam Combination) ...........34
Other Pre-Euthanasia Drugs: Conditionally Acceptable, but Not Preferred
...................35
Administration of Pre-Euthanasia Drugs ..................................................37
Inhalant Anesthetics (Halothane, Isoflurane) .............................................39
CHAPTER 4:
Verification of Death—The Most Critical Step in the Euthanasia Process .......41
Verifying Death .............................................................................42
Performing a Cardiac Stick (‘Heart Stick’)
....................................................43
Table of Contents

iv
The Humane Society of the United States Euthanasia Reference Manual
CHAPTER 5:
Disposal of Animal Bodies ................................................................45
CHAPTER 6:
Euthanasia Tools .............................................................................47
The Euthanasia Area ........................................................................47
Layout and Design
.........................................................................47
Lighting and General Environment .........................................................48
Recommended Equipment .................................................................48
Syringes
...................................................................................49
Needles
.................................................................................... 50
Sharps Containers
..........................................................................51
Tourniquets/Hemostats
....................................................................51
Scale
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 52
Electric Hair Clippers
.......................................................................52
Other Recommended Supplies .............................................................52
CHAPTER 7:
Euthanasia Policy and Protocols .........................................................54
Selection Criteria ............................................................................54
Policy Elements .............................................................................56
Volunteers and Euthanasia .................................................................57
CHAPTER 8:
Animal Handling and Restraint ..........................................................58
Typical Restraint Techniques—Dogs .......................................................58
Restraint for Direct Injection
................................................................ 58
Restraint for Administration of Pre-Euthanasia Drugs .......................................59
Typical Restraint Techniques—Cats ........................................................60
Restraint for Direct Injection ................................................................60
Restraint for Administration of Pre-Euthanasia Drugs
.......................................60
General Restraint Techniques ..............................................................61
Restraint Tools ..............................................................................61
Time
....................................................................................... 62
Leash
......................................................................................62
Towel
...................................................................................... 62
Gloves
.....................................................................................63
Muzzle ....................................................................................63
Press Gate
.................................................................................63
Control Pole
...............................................................................64
Table of Contents

v
The Humane Society of the United States Euthanasia Reference Manual
Squeeze Cage ..............................................................................65
Feral Cat Box
...............................................................................66
Cat Bags/Nets ..............................................................................66
Cat Graspers/Tongs
........................................................................66
CHAPTER 9:
Employee Health and Safety
..............................................................67
Bites/Scratches ..............................................................................67
Needle Sticks ................................................................................68
Eye Injuries ..................................................................................68
General Hazards ............................................................................69
Compassion Fatigue/Euthanasia-Related Stress ..........................................69
CHAPTER 10:
Federal Requirements for Controlled Substances
...................................71
Storage ......................................................................................72
Record-Keeping Requirements ............................................................73
Inventory Records ..........................................................................74
Reporting Theft/Loss .......................................................................74
Training of Euthanasia Technicians ........................................................74
CHAPTER 11:
Unacceptable Methods of Euthanasia
..................................................75
CHAPTER 12:
Euthanasia of Other Animals .............................................................76
Small Mammals .............................................................................76
Rabbits ....................................................................................76
Small Rodents (Mice, Rats, Hamsters,
Gerbils, etc.) ...........................................76
Guinea Pigs ................................................................................77
Ferrets
..................................................................................... 77
Birds .........................................................................................77
Reptiles ......................................................................................78
Snakes
.....................................................................................78
Turtles, Tortoises, and Terrapins .............................................................79
Crocodilians (Alligators and Crocodiles)
.....................................................79
Lizards
..................................................................................... 79
Fish ..........................................................................................80
Table of Contents

vi
The Humane Society of the United States Euthanasia Reference Manual
Amphibians .................................................................................80
Large Domestic Mammals ..................................................................80
Equines (Horses, Donkeys, Mules)
...........................................................81
Ruminants (Cows, Goats, Sheep)
............................................................ 81
Pigs
........................................................................................81
Wildlife ......................................................................................81
Bats
.......................................................................................82
Deer, Elk, and Other Large Hooved Animals .................................................83
Bears, Coyotes, Mountain Lions, Primates, and Other Large Mammals. . . . . . . . . . . . . . . . . . . . . . . . 83
CHAPTER 13:
Field Euthanasia .............................................................................84
Confinement for Field Euthanasia .........................................................85
Field Euthanasia by Injection of Sodium Pentobarbital ...................................85
Field Euthanasia by Gunshot ...............................................................86
Types of Firearms ..........................................................................86
Correct Shot Location by Species
...........................................................88
Disposal
...................................................................................89
CHAPTER 14:
Mass Euthanasia .............................................................................90
Glossary .......................................................................................91
Dosage Chart for Telazol® and PreMix (ketamine/xylazine combination) ................93
Injection Methods—Quick Reference ...................................................94
Index...........................................................................................95
Table of Contents

vii
The Humane Society of the United States Euthanasia Reference Manual
Acknowledgments
The previous edition of this manual was authored by Rebecca H. Rhoades, DVM, and we are
grateful for her continued involvement and review of this updated edition. We would like to
oer our sincere thanks to the other experts who participated in the review of this edition:
Dr. Wendy Swift, Dr. Martha Smith, Dr. Mark Jones, and Douglas Fakkema.
We would also like to acknowledge the contributions of sta from The HSUS, HSVMA and
HSI, including Inga Fricke, Catherine Lynch, Betsy McFarland, John Haddidian, Hilary Hager,
Pam Runquist, and Kelly Coladarci.
Last but not least, The HSUS wishes to acknowledge and thank the many animal shelter and
rescue sta and volunteers who are working tirelessly to save lives and make euthanasia of
healthy and treatable animals a tragedy of the past.
Portions of this work were originally published by the Humane Society of Willamette Valley,
in Salem, Oregon, as Handbook of Pentobarbital Euthanasia by Tim Greyhavens (copyright
1989 by The Humane Society of the Willamette Valley).
All illustrations by Les Sealing unless otherwise noted.
Acknowledgments

viii
The Humane Society of the United States Euthanasia Reference Manual
Foreword
Since the first edition of this manual was published, in 2002, not much has changed
in terms of “how” euthanasia is performed; the debate over “why,” on the other
hand, has become more contentious than ever. Over the last few decades, euthanasia
numbers have declined sharply—from over 23 million cats and dogs euthanized in
1970 to fewer than 4 million in 2010, even as pet ownership rose steadily over that
period. As we come ever closer to achieving zero euthanasia, the challenges become
greater, and the debate over which animals must be euthanized and which can be
saved seemingly grows more intense. Euthanasia technicians should welcome this
debate—as a profession, we should be clear, transparent, and honest about our
decisions and agree that euthanasia should ultimately be reserved only for animals
that are suering or are too aggressive to safely place in homes.
Regardless, we must ensure that when euthanasia is performed it is as humane
as possible. Direct injection of sodium pentobarbital (referred to as euthanasia by
injection, or EBI) remains the most humane method available. We are encouraged
to report that more organizations than ever before have adopted EBI, as opposed
to using far less appropriate alternatives like gas chambers. It is our hope that by the
time the next edition of this manual is published we will have reached the point where
every shelter practices only the most humane methods of euthanasia available.
No one wants to perform euthanasia, but the people who take on this emotional
and unwelcome task owe it to the animals to do it well. We hope this manual will
serve as the definitive basic education tool for understanding the methods of
humanely ending an animal’s life; however, it cannot provide answers to the topic’s
moral questions. Euthanasia technicians should use this manual to refine their skills,
but they must also develop programs and initiatives intended to bring an end to
the need for euthanasia of healthy and treatable animals.
Foreword

1
The Humane Society of the United States Euthanasia Reference Manual
CHAPTER 1
Understanding Euthanasia
What Is Euthanasia?
Euthanasia involves more than ending an
animal’s life. It is a process that combines
compassion and scientific consideration while
providing each animal with a death that is free
of pain and stress. Along with the technical
skills required, there must be compassion and
a sense of solemnity, reverence, and respect
for the animals.
Humane euthanasia of an animal requires five
basic elements:
1. Compassion.
2. Knowledge.
3. Technical skills developed through
training and experience.
4. Appropriate application of the most
state-of-the-art drugs, equipment, and
techniques available.
5. Wisdom to know when euthanasia
should, and should not, be performed.
This manual will bring these five attributes into
focus so that those entrusted with the care of
sheltered animals have the knowledge, skill,
equipment, and insight to serve their needs,
even if this means ending their suering
through euthanasia.
Euthanasia, Past
and Present
Euthanasia, simply defined, is the act of induc-
ing a painless death. The word itself comes
from the ancient Greek “euthanatos”—eu
meaning easy, and thanatos meaning death.
Providing a “good death” for shelter animals
whose lives must be ended should without
a doubt be uppermost in the minds of those
entrusted with the task. In the 19th and early
20th centuries, long before pain-free methods
of ending life were available and when the pri-
mary reason for destroying dogs and cats was
not overpopulation but the control of rabies,
such a goal was virtually unattainable. As the
threat of rabies and other diseases diminished,
the population of domestic pets skyrock-
eted, and with this increase in numbers came
various methods for eliminating homeless
animals; sadly, humane considerations were
often secondary.
Over the years, many techniques were tried in
search of “better” methods. Tragically, drown-
ing was one of the earliest methods used; in
many cities at the turn of the 20th century,
animals were loaded into huge cages and lifted
by cranes into a river or bay. Electrocution
was popular in the 1920s, with animals placed
in cages with zinc floors wearing conductive
collars. With the advent of the gasoline engine,
the use of carbon monoxide “gas chambers”
became commonplace. This method was later
refined by replacing the raw, hot exhaust of an
engine with commercially prepared gas from
tanks. The decompression chamber was consid-
ered an improvement over previous methods
when it was initially developed; however,
persistent mechanical problems and questions
about its humaneness quickly prompted bans
in most areas, and the method is completely
eliminated today.
Since 1963, the American Veterinary Medical
Association (or AVMA) has reviewed eutha-
nasia methods by regularly gathering a panel
of experts to evaluate the latest studies and
information about the various methods
available. The report, updated most recently
Understanding Euthanasia

2
The Humane Society of the United States Euthanasia Reference Manual
in 2013 (at the time of this manual’s publi-
cation), contains a list of recommendations
intended for veterinarians (and by extension,
trained euthanasia technicians) to follow in
providing euthanasia for a variety of species.
The AVMA report is the most extensive study
of euthanasia methods currently available.
The association’s panel evaluates each method
based on 14 criteria:
1. ability to induce loss of consciousness
and death with a minimum of pain
and distress;
2. time required to induce loss
of con sciousness;
3 reliability;
4. safety of personnel;
5. irreversibility;
6. compatibility with intended ani mal
use and purpose;
7. documented emotional e ect on
observers or operators;
8. compatibility with subsequent
evaluation, examination, or use
of tissue;
9. drug availability and human
abuse potential;
10. compatibility with species, age,
and health status;
11. ability to maintain equipment in
proper working order;
12. safety for predators or scavengers
should the ani mal’s remains
be consumed;
13. legal requirements; and
14. environmental impacts of the method
or dis position of the animal’s remains.
Since 1986, the AVMA has consistently con-
cluded that “the intravenous injection of
barbituric acid derivatives [i.e., sodium pento-
barbital] is the preferred method for euthanasia
of dogs, cats, other small animals, and horses.”
When administered properly, sodium pento-
barbital is capable of causing death quickly and
painlessly (criteria 1 and 2) and may be used
consistently and reliably with many species
(criteria 3, 5, 6, and 10).
Nevertheless, even sodium pentobarbital
does not meet all 14 criteria. For example, it
is a federally controlled substance with high
abuse potential, which puts it at odds with
No. 9. Moreover, it has several characteristics
that may be considered both advantages
and disadvantages. Its use requires close
contact with each animal, which can provide
beneficial comfort and the reduction of
stress to the animal (No. 6), but as with all
euthanasia methods, this takes a toll on
the humans involved (although technicians
performing euthanasia by injection are
generally heartened by their ability to gently
hold the animals, reporting less stress than
those who use more removed methods like
the gas chamber). Eective use of sodium
pentobarbital also typically requires the
participation of at least two trained sta
members, to provide a higher degree of safety
and support for the personnel involved (No.
4); some view this as a disadvantage because
dedicating multiple sta members to the
procedure requires more time than methods
that can be performed by one person, but a
properly trained team can actually be more
ecient than a single technician.
While sodium pentobarbital may not be
the perfect means of euthanasia, it comes
closest of any available methods. All of its
disadvantages weigh against the people
who administer it, and all of its advantages
weigh in favor of the animals. Therefore, its
deficiencies, while certainly legitimate, do not
outweigh its advantages, and should not be
used as an argument in favor of less accept-
able alternatives.
Understanding Euthanasia

3
The Humane Society of the United States Euthanasia Reference Manual
We hope that in the future, an even more
sophisticated technique will be developed—
one that meets all 14 of the AVMA’s criteria.
In the meantime, it is our responsibility to use
the best method we have, and to use it with
skill, compassion, and consistency.
Important Definitions
Before discussing euthanasia techniques it is
vital to establish a common understanding of
the language of euthanasia. It is not unusual
to hear people use terms like sedation, tran-
quilization, and anesthesia interchangeably.
However, in order to achieve the most humane
death possible for each animal by selecting the
proper drugs it is imperative to understand
their dierences:
Consciousness: When conscious, an animal
has the ability to deliberately and intentionally
respond to environmental stimuli.
Unconsciousness: When rendered uncon-
scious, the animal lacks awareness and the
capacity for sensory perception, appearing
to be in a deep sleep.
Tranquilization: When tranquilized, the
animal usually is calm and relaxed, and he
may even fall asleep. The animal may still
feel pain, however, and a tranquilizer may
not oer enough of a calming eect to safely
handle a fractious animal. Tranquilized animals
may also suer seizures, and can be
more unpredictable.
Sedation: When sedated, the animal falls into
a sleep-like state and becomes uncoordinated,
with relaxed and unresponsive muscles. There is
often a decreased ability to feel pain, but pain
sensations are still possible. Sedated animals
may appear to be sleeping but may quickly
become aroused when stimulated by light or
sound and cause harm to themselves and the
humans around them.
Immobilization: When immobilized, the animal
is essentially paralyzed and unable to move.
However, while the animal appears to be unre-
sponsive to sight and sound, he may still feel
deep pain and may actually be experiencing
fear and panic as he remains aware of his sur-
roundings. For this reason, immobilizing agents
are never appropriate for use in euthanasia.
Analgesia: Drugs that have an analgesic eect
are intended to diminish an animal’s ability to
perceive pain, although not all drugs can extin-
guish pain completely. Moreover, just because
a drug has an analgesic eect does not mean
that it causes unconsciousness in the animal.
Therefore, the ideal pre-euthanasia drug
is both an analgesic and an anesthetic.
Anesthetic: When an anesthetic agent has
been administered, the animal is ideally ren-
dered unconscious, has a total loss of ability
to feel pain (analgesia), and is immobilized,
yet her vital functions (breathing and heart-
beat) are retained. For this reason, the ideal
pre-euthanasia drugs are anesthetics that,
when used at proper dosages, achieve all
of these ideals.
Understanding Euthanasia

4
The Humane Society of the United States Euthanasia Reference Manual
CHAPTER 2
Sodium Pentobarbital
Sodium pentobarbital belongs to a large family
of drugs known as barbiturates. First used
clinically about the turn of the 20th century
as medical hypnotics, barbiturates like sodium
pentobarbital served as the basis for many
types of anesthetic agents now used for human
and veterinary surgery. When used as surgical
anesthesia, a barbiturate is dosed to safely
cause short-term unconsciousness and loss of
pain for the required duration. When used for
euthanasia, barbiturates in general, and sodium
pentobarbital in particular, are used at doses
higher than would be used in surgery in order
to quickly and completely depress the animal’s
central nervous system, resulting in death.
How Sodium
Pentobarbital Works
Sodium pentobarbital is an eective euthanasia
agent because of its eect on brain function.
When introduced into the bloodstream, sodium
pentobarbital moves rapidly to the heart and
then into the brain, where it quickly and pain-
lessly depresses all vital life functions. A lethal
intravenous dose of sodium pentobarbital causes
a mammal to lose consciousness within seconds,
and results in clinical death within just minutes.
To understand how the drug works, why it is
considered to be the most humane method of
euthanasia for virtually all animals, and how
to recognize and correct potential problems
after the drug has been administered, one
must understand the stages of anesthesia that
each animal undergoes once the drug has been
administered. Each stage is identified by physio-
logical changes within the animal’s body. While
sodium pentobarbital typically works so quickly
Sodium pentobarbital is commercially
available in two variations, either alone
or in combination with a drug called
phenytoin sodium (an anticonvulsant drug
that is intended to decrease abnormal
electrical activity in the brain). Sodium
pentobarbital alone (either in liquid form
or in a powder that must be reconstituted
with water) is available under trade names
like Fatal-Plus, Euthanasia-6, Pentasol, and
Succumb, and can be used for virtually all
species of animals. However, because of
its higher concentration of barbiturate
(with its associated mood-altering
properties) it has a high risk of addiction
and fatal overdoses in humans; therefore,
in this form sodium pentobarbital is
strictly regulated by federal and state
laws (see Chapter 10). When combined
with phenytoin sodium, however (under
trade names like Euthasol, Beuthansia,
and Euthanasia III), the drug loses its
properties as an addictive substance
(the phenytoin sodium actually serves to
hasten cardiac arrest, making the drug
unsuitable for achieving a safe “high”)
and is therefore not subject to the same
stringent acquisition controls. However,
these combined formulations are
approved for use only in dogs, and their
routes of administration are restricted
(these formulations may not be injected
into the abdominal cavity, for example).
Eorts are under way to produce
formulations that combine sodium
pentobarbital with lidocaine, though none
is commercially available yet. IMPORTANT
NOTE: Formulas that combine sodium
pentobarbital with any other agent
besides phenytoin sodium or lidocaine
are not acceptable for use in euthanasia.
Sodium Pentobarbital

5
The Humane Society of the United States Euthanasia Reference Manual
and eectively that it can be dicult to discern
when the animal is moving from one stage to
another, it is nevertheless crucial for euthanasia
technicians to understand the process in order
to appreciate how the drug so successfully
provides a humane death.
Stage I: Voluntary Excitement. As the sodium
pentobarbital is injected, the drug makes its
way from the injection site to the heart, and
is then pumped to the outermost layer of the
brain called the cerebral cortex. As the animal
begins to lose consciousness and coordination,
he or she may become increasingly sensitive
to noise, touch, and other stimuli. Gentle, safe
restraint and a quiet environment are essential
to minimize excitement during this phase. As
the drug passes through the cerebral cortex,
the animal quickly begins to lose the ability
to feel pain (although he or she can still feel
“deep” pain) and loses voluntary motor skills.
IMPORTANT NOTE: Euthanasia technicians
should be aware that when sodium pentobarbi-
tal moves through the brain it also shuts down
the animal’s normal inhibitory centers, which
typically keep the animal from biting; when
this happens, the disorientation the animal is
experiencing can cause even the gentlest soul
to react. Luckily, sodium pentobarbital works
so quickly that euthanasia technicians are rarely
hurt, but it is important to be aware of the
possibility and keep the animal safely restrained
until complete loss of consciousness occurs.
Stage II: Involuntary Excitement. Next, the
drug moves through the cerebral cortex into the
cerebrum, the area of the brain responsible for
higher-order functioning, like senses, memory,
personality, and emotions. The imbalance result-
ing from the presence of the drug in this region
frequently causes uncontrolled motor activity like
paddling of the legs and vocalizations; although
this may be disconcerting to see, the animal is
completely unconscious and not suering.
Stage III: Surgical Anesthesia. Once the drug
has made its way through the cerebrum it enters
the cerebellum, the area of the brain that governs
balance and gross motor activity. At this point
the animal can feel no pain at all and does not
respond to visual or auditory stimuli, and reflexes
(including eye blink and toe-pinch) begin to
disappear. Now the animal is considered to be in
a state of surgical anesthesia and could be safely
and painlessly operated on (provided vital signs—
heartbeat and respiration—are maintained). If
sodium pentobarbital has been injected into a
Typical euthanasia drugs
Sodium Pentobarbital
How fast does sodium
pentobarbital work?
Typically, within five seconds after injec-
tion into a vein the animal is unconscious,
within 20 seconds the animal stops
breathing, within 40 seconds the animal
is considered “medically dead” (meaning
the heart has ceased circulating blood),
and within two minutes the animal is
“clinically dead” (meaning all voluntary
and involuntary functions have ceased,
although muscle twitching may continue
for several minutes).

6
The Humane Society of the United States Euthanasia Reference Manual
vein, the animal generally reaches this stage as
soon as four to five seconds after injection.
Stage IV: Medullary Paralysis. When perform-
ing surgery on an animal in Stage III anesthesia,
specialists ensure that the drugs administered
do not compromise the core functions of the
deepest part of the brain, in the brain stem
(medulla oblongata). The brain stem is responsi-
ble for the body’s most basic needs for survival,
namely breathing, heartbeat, and blood pres-
sure. With euthanasia, however, the goal is to
have the drug reach the animal’s brain stem and
depress these core functions as quickly as pos-
sible. Sodium pentobarbital achieves this goal,
stopping the animal from breathing in oxygen
and circulating blood usually within just 40 sec-
onds after injection directly into a vein. When
these core functions cease, the animal dies.
It is important to understand that even after
the core functions have ended and death has
occurred, the body may appear to the untrained
eye to be showing signs of life—for example, the
animal’s heart may fibrillate, or the animal may
audibly appear to gasp (called agonal breathing).
These are not conscious, voluntary responses
by the body or indications that the animal is
“fighting for life.” For example, heart fibrillations
are little more than muscle spasms; they do not
indicate that the heart is eectively circulating
blood. Similarly, agonal breathing is simply the
body’s reflexive attempt to address its shortage
of oxygen; the animal is not actually breathing,
or trying to breathe, in a manner that would
sustain life. Knowing the dierence between
actual life signs and involuntary death responses
is a critical part of performing euthanasia.
IMPORTANT NOTE: It is not enough to simply
inject an animal with a lethal dose of sodium
pentobarbital and assume that he or she has
been euthanized. Euthanasia is not complete
until death has been verified (see Chapter 4).
If sodium pentobarbital has been injected
directly into a vein, the animal typically passes
through all four stages of anesthesia in a mat-
ter of seconds; if the drug has instead been
injected into the abdominal cavity, the process
will take longer simply because the drug must
first be absorbed into the bloodstream in
order to then be carried from the heart to the
“Lethal dose” vs. “label dose”
A lethal dose of sodium pentobarbital
is the amount of drug sucient to move
the animal through all four stages of
anesthesia and stop the core functions
of life (respiration and circulation). Because
sodium pentobarbital is intended to serve
as a euthanasia agent, the actual amount
of drug administered when following the
dosage on the label (the “label dose”) is
much higher than the amount necessary
to achieve death (the “lethal dose”)—30%
to 50% higher. This “extra” drug is intended
to serve as a cushion to ensure that if the
proper amount of drug is administered in
the proper manner, the animal will in fact
die humanely. IMPORTANT NOTE: This
does not eliminate the need to arma-
tively verify death, as a variety of factors
can influence how much drug the body
actually absorbs.
Sodium Pentobarbital
The anatomy of the brain
Cortex
Cerebrum
Cerebellum
Brain Stem

7
The Humane Society of the United States Euthanasia Reference Manual
brain. Regardless of method, a well-trained,
experienced euthanasia technician can rec-
ognize when an animal is not following the
typical timeline for the stages of anesthesia,
and make necessary corrections to the process.
Administering Sodium
Pentobarbital
Sodium pentobarbital may be administered
to an animal in several ways: IV (intravenous,
injection of drug directly into a vein), IP (intra-
peritoneal, injection of the drug into the
abdominal cavity), IC (intracardiac, injection of
the drug directly into the heart), or PO (per os,
oral administration of the drug). Each method
has advantages and disadvantages. A qualified
euthanasia technician should be comfortable
with each, and should select the method that
will provide the most humane euthanasia for
the animal under the circumstances.
Intravenous (IV) Injection
(Injection of Sodium Pentobarbital
Directly into a Vein)
With this method, sodium pentobarbital is
directly injected into the animal’s vein, where
the drug is carried by the circulatory system to
the heart and then on to the brain. Once the
proper quantity is injected, loss of conscious-
ness is nearly instantaneous, and death quickly
Sodium Pentobarbital
Supply List
Before picking up a needle for injection,
a euthanasia technician should ensure
that all of the supplies necessary to
perform euthanasia and manage any
complications are at the ready, including:
• Electric clippers
• Tourniquet/hemostat
• Needles of various sizes
• Syringes of various sizes
• Towels/blankets
• Muzzle, net, press gate, or other
appropriate humane restraint devices
• A “backup” syringe with sucient
amount of pre-euthanasia anesthetic
to render the animal unconscious
A well-trained euthanasia technician must
determine the most appropriate method,
based on the animal’s species, age, medical
condition, and temperament.
Intravenous (IV)
(injection of drug directly into a vein)
Primary Advantages
• Can be used on virtually any animal,
regardless of age or species.
• Can be used on conscious or
unconscious animals.
• Appropriate veins are typically
readily accessible.
• Causes minimal distress or pain
when proper technique is used.
• Results in rapid death, as the drug
is introduced directly into the
circulatory system.
• Allows technicians to hold and
comfort the animal.
Primary Disadvantages
• The close contact required for direct
injection into conscious animals can
cause undue stress for some animals
or can put technicians at undue risk
of injury if the animal is fractious.
• The animal’s medical condition can
make locating and injecting into
veins a challenge.
• It generally cannot be used on very
tiny animals whose veins are too small
for injection.
8
The Humane Society of the United States Euthanasia Reference Manual
follows. Because there are no nerve endings
inside veins, the only pain an animal feels in con-
nection with IV injection is the initial prick of the
needle piercing the skin. With education, train-
ing, and practice, a euthanasia technician can
become extremely proficient at minimizing this
needle sting and can “hit” (locate and enter) the
vein on the first try virtually every time, creating
a very humane and nearly painless experience.
IV injection is the most flexible type of injection
in that it can be used on virtually any animal,
with few restrictions for age or health. It also
has the unique advantage of being appropriate
for use on conscious dogs, because it is virtually
painless and because it does not require
handling that most dogs find uncomfortable
or invasive. Well-socialized dogs can be gently
restrained while a euthanasia technician injects
the drug directly into a leg vein (for this reason,
IV injection into a conscious animal is often
referred to as “direct injection”); using this
direct injection method, the dog receives the
benefit of close, comforting human contact
in the last moments and avoids the pain that
accompanies the injection of most pre-eutha-
nasia drugs (see Chapter 3). For this reason, the
preferred method for humanely euthanizing
well-socialized dogs is direct IV injection of
sodium pentobarbital without administration
of any pre-euthanasia drug.
Selecting Veins for IV Injection
IV injection can be performed on any of sev-
eral veins throughout an animal’s body. When
selecting a vein, a euthanasia technician should
take into account factors like species, size, and
Sodium Pentobarbital
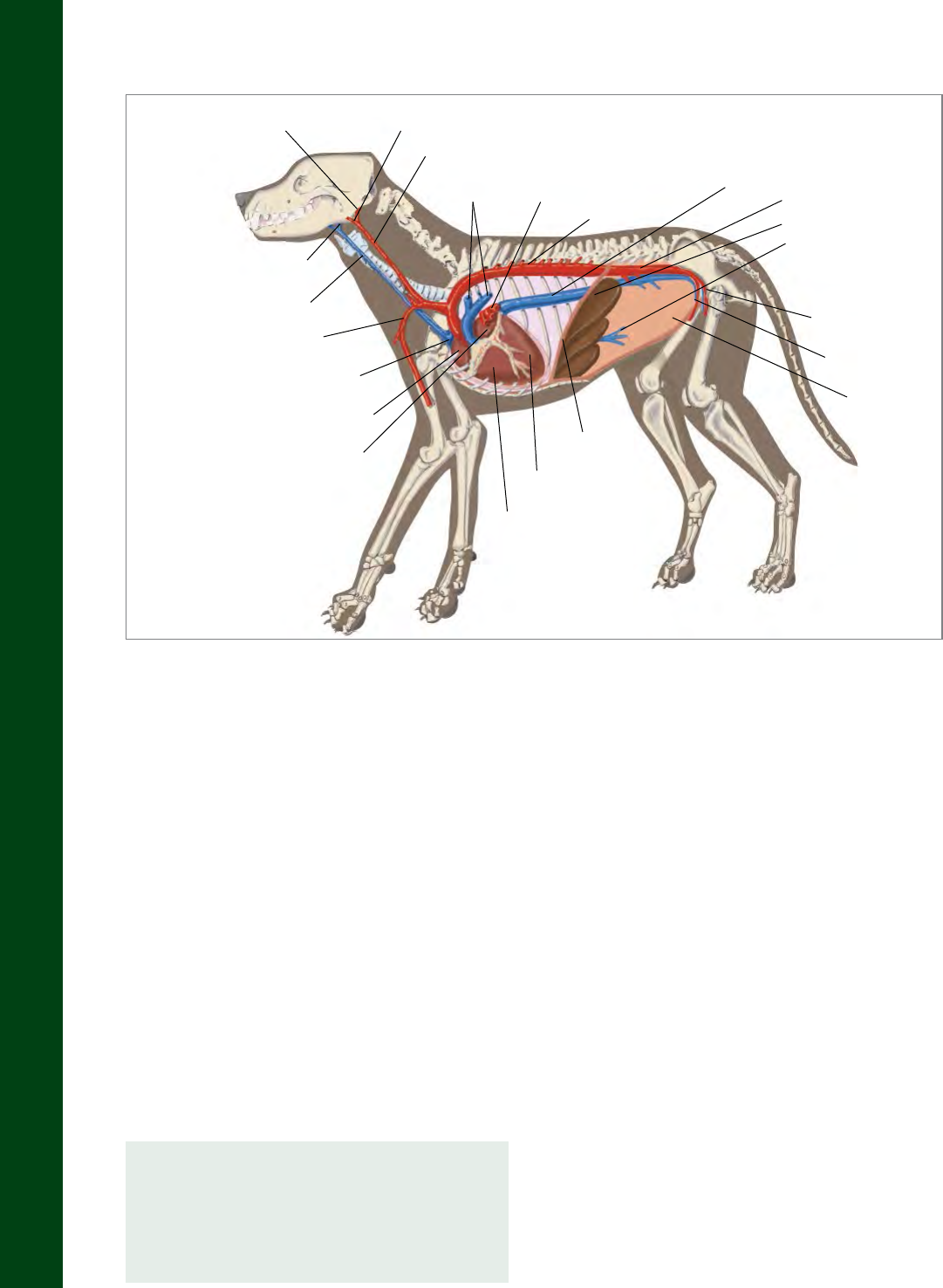
Circulatory system of a dog
Internal Carotid Artery External Carotid Artery
Common Carotid Artery
Pulmonary
Arteries
Pulmonary
Veins
Thoracic Aorta
Caudal
Vena Cava
Abdominal Aorta
Liver
Hepatic Portal Vein
Femoral Artery
Femoral Vein
Abdomen
Diaphragm
Left Ventricle
Right Ventricle
Left Atrium
Right Atrium
Cranial Vena Cava
Brachial Artery
External Jugular Vein
Internal Jugular Vein
Cats tend to resist even the gentle restraint
associated with IV injection, so IP (intraper-
itoneal) injection is often preferred over IV
for conscious, well-socialized cats.

9
The Humane Society of the United States Euthanasia Reference Manual
whether the animal will be conscious or uncon-
scious when the injection is given. Another
important consideration is the technician’s
personal preference and comfort level—some
people are simply more adept at “hitting” veins
(successfully administering IV injection) in an
animal’s front legs than back legs, or vice versa.
Any vein can be acceptable provided accessing
it does not place any undue stress on the animal
(assuming the animal is conscious). Whichever
veins are preferred, however, euthanasia
technicians must be able to locate and inject
into any of them, because the animal’s front
or rear leg veins may be unavailable because
of illness or injury, and because even the most
skilled technicians can “blow” veins (see dis-
cussion on Injection, below), requiring them
to move from one leg to another to achieve
successful injection.
Four pairs of veins can be used for IV eutha-
nasia injections in mammals (the trained
euthanasia technician must determine
which is most appropriate):
The cephalic veins run prominently down the
front of each foreleg of an animal. They are a
preferred route for direct injection of conscious,
socialized, friendly dogs because the handler
can easily extend the foreleg while providing
gentle, humane restraint. The cephalic veins
also are held tightly in place and tend not to
“roll,” or move sideways within the leg, quite
as easily as other veins can. Many technicians
prefer them for these reasons.
The lateral saphenous veins run down the
outside of the animal’s rear legs and then cross
diagonally across the leg just above the hock.
The lateral saphenous veins of adult dogs tend
to be fairly large and easy to find, making
them a preferred choice for many technicians,
provided they choose their injection location
appropriately. Lateral saphenous veins must
Sodium Pentobarbital
Cephalic vein of a dog Lateral saphenous vein of a dog
Cephalic Vein
Lateral Saphenous
Vein
Accessory (Auxiliary)
Cephalic Vein
Throughout this manual, the term “han-
dler” will be used to refer to the person
holding the animal for euthanasia and the
term “technician” will be used to refer to
the person performing the injections.

10
The Humane Society of the United States Euthanasia Reference Manual
be injected away from the hock joint or they
can roll, and they do not run directly from top
to bottom of the leg as the cephalic veins do.
The lateral saphenous vein can be used for
direct injection of conscious dogs, as long as
the animal is not unduly stressed by the gen-
tle restraint of its rear leg. Cats, puppies, and
kittens, however, tend to have small lateral
saphenous veins, making these veins a less
attractive choice.
The medial saphenous veins (aka femoral
veins) run straight down the center of the
inside of the animal’s rear legs. They can be
a good choice for unconscious cats, whose
lateral saphenous veins may be too small to
easily inject into.
The jugular veins run down each side of the
neck of most mammals. Restraining a cat or a
dog safely for jugular injection can be dicult,
causing undue stress on both the animal and
the handler. Moreover, the jugular vein in an
animal as small as a dog or cat is very close to
the trachea and carotid arteries, increasing
the likelihood of improper injection. For these
reasons, injection into the jugular vein of a dog,
cat, or other small mammal should be avoided.
For large animals like horses, however, the
jugular veins are readily accessible and clearly
evident, making injection fairly easy . Moreover,
in these large animals it is often impractical
or dangerous to try to inject veins in the leg,
making jugular injection the safest option for
the technician.
Administering IV Injections
NOTE: The following describes the correct
procedure for injecting into a cephalic vein,
but this basic technique is also generally
applicable to injections into other veins
as well; specific information pertinent to
injecting into the saphenous and jugular
veins follows.
Readying the Syringe and Needle
Regardless of the method used to inject sodium
pentobarbital, selection of the appropriate
syringe and needle is the first step to ensur-
ing success. The volume of drug required will
dictate the size of the syringe. For the most
part, the larger the syringe the more unwieldy
it is; therefore, technicians should always select
Sodium Pentobarbital
View of the medial saphenous vein of a cat
Location of the jugular veins in a horse
Medial Saphenous
Vein
Femoral Vein
THE HSUS

11
The Humane Society of the United States Euthanasia Reference Manual
the smallest syringe capable of handling the
necessary dose of drug (see Chapter 6 for more
information on syringes).
By contrast, the needle will depend primarily on
the size of the animal. Needles are available in
several lengths and sizes, called gauges, accord-
ing to the bore diameter of the needle. It may
appear counterintuitive, but the smaller the
diameter of the needle, the larger the gauge
size—for example, a 25-gauge needle is smaller
than a 22-gauge needle, which is smaller than
an 18-gauge needle. Choosing a smaller nee-
dle generally produces less discomfort for the
animal on injection; however, if a large volume
of drug is required, a smaller needle may be not
be able to deliver the necessary volume of drug
at an appropriate pace, or may fail completely
and pop o the end of the syringe. Selecting
the proper needle may seem to be a fairly minor
consideration, but it is a vital part of a good
euthanasia technician’s toolkit for making
euthanasia as humane as possible.
Once the proper needle and syringe have been
selected, the technician should insert a spare
needle into the bottle and draw up the appro-
priate amount of sodium pentobarbital into the
syringe, then attach the needle. Once that task
is completed, both the euthanasia technician
and the handler should verify one last time that
they have the right animal before them, that
the appropriate paperwork authorizing eutha-
nasia is in order, and that the animal has been
scanned once more for a microchip or other
identification. Once all proper verifications
have been made, injection can proceed.
Sodium Pentobarbital
While needles must never be reused,
syringes can be. It is critical, however, not
to use the same syringe to inject first one
drug and then another (for example, do
not use a syringe for administering first
a pre-euthanasia drug and then sodium
pentobarbital), since any leftover drug
remaining in the syringe can crystallize
and cause obstruction. Moreover, blood
left in the syringe can interfere with the
technician’s ability to see the flash of
blood that shows the needle is properly
in the vein. For these reasons, it is rec-
ommended that new syringes be used at
least during every new euthanasia ses-
sion, and that separate syringes be used
for each separate drug administered.
Readying for injection into the cephalic vein
The seals of sodium pentobarbital bottles
are quite durable and will dull any needle
pushed through them. For that reason,
needles that have been pushed through
a sodium pentobarbital bottle top should
never be used to pierce the skin of an ani-
mal, since a dull needle means increased
pain. Most technicians insert a single
needle (usually mid-to-large gauge) into
the sodium pentobarbital bottle at the
start of each euthanasia session, and use
that same needle to draw all drug into the
syringes. It is not necessary to use a new
needle for every draw of drug.

12
The Humane Society of the United States Euthanasia Reference Manual
Locating the Proper Injection Site
Assuming the euthanasia technician has
decided to inject sodium pentobarbital into the
cephalic vein (regardless of whether the animal
is conscious or not), the handler should gently
extend the animal’s foreleg (see Chapter 8 for
information on humane restraint) so that the
front of the leg can be shaved. Shaving is not
an absolute necessity, but it gives the techni-
cian a clearer view of the vein and increases
the odds the injection will succeed on the first
attempt. The technician should use clean and
well-lubricated electric clippers (size 40 blades
are recommended) to shave a sucient area
over the front of the animal’s leg to allow
access to the vein, clipping opposite the direc-
tion of hair growth (from paw to shoulder) to
produce the closest shave. Care should also be
taken to avoid “clipper burn,” abrasion of the
skin caused by excessive heat from the friction
of the clipper blades or poor blade position-
ing; holding the clipper at the proper angle
(typically flush against the skin), applying only
light, consistent pressure, and ensuring proper
lubrication of the blade will avoid this. Some
technicians prefer not to shave, since the use
of noisy clippers may add to an animal’s stress;
however, a higher degree of skill is necessary
to locate the vein without first shaving the
area. As an alternative, curved scissors may
be used to eliminate excess fur, although care
must be taken as the skin may “tent up” and
be accidentally cut.
The technician may sprinkle water on the area
to help make the vein more visible—not only
does the presence of water flatten the hairs
on the leg, the evaporation process helps
to tighten the skin surface making the vein
appear more prominent. Alcohol should not
be used on a conscious animal if the fur has
been shaved, since it causes an uncomfortable
stinging sensation.
In some animals, once the hair has been
removed, the vein will be easily visible as a
long, bluish line running down the front of
the leg. However, in most cases, an additional
step, known as “holding o the vein,” must
be taken to make the vein accessible for
injection. Veins carry blood to the heart; in
the case of the cephalic veins, blood is being
pumped continuously up the leg from the paw
toward the shoulder. When the flow of blood
is constricted, the blood below the point of
constriction backs up, increasing the pressure
in the vein; this increase in blood pressure
causes the vein to enlarge, making it much
easier to see and feel (imagine a cheap garden
hose—if the hose develops a kink that prevents
The proper angle for gentle shaving of the injection site
Sodium Pentobarbital
Some euthanasia technicians mistakenly
believe that alcohol must be sprayed on
the injection site to “raise” the animal’s
vein, making it easier to inject. However,
in many cases the alcohol only dampens
the hair, giving the illusion that the vein
is raised. Moreover, the alcohol can cause
a burning sensation and can make the
needle puncture more painful for the
animal, particularly if the leg has been
shaved. Technicians should therefore never
apply alcohol to the shaved legs of conscious
animals before injection (for technicians
reliant on spraying alcohol, water can be
used as an eective alternative).

13
The Humane Society of the United States Euthanasia Reference Manual
water from continuing to move through,
the pressure will cause the hose to appear
to expand just below the point of the kink).
This eect (referred to as “raising the vein”)
can be achieved either by having the handler
“hold o” the vein manually or by applying
a tourniquet or other mechanical means of
blocking o the vein.
To hold o the cephalic vein, the handler grasps
the leg from behind, “cupping” the animal’s
elbow in his or her palm, then wraps her thumb
over the vein and applies firm, consistent
pressure. This approach gives the handler a con-
trolling grasp that not only obstructs the blood
flow of the cephalic vein, causing it to swell so
that it can be injected into more easily, but also
helps to gently prevent the dog from retracting
his or her leg away from the injection. The han-
dler may roll her thumb and hand (along with the
skin and vein it holds) slightly outward so that the
vein is positioned directly on top of the leg bone,
creating the ideal position for injection.
If the handler requires both hands to gently
restrain the animal or is otherwise unable to
adequately hold o the vein, a commercially
purchased tourniquet system may be used
to achieve the same result. A Nye tourniquet
consists of strong rubber cord that is placed
around the animal’s leg and then is clamped
with an attached friction grip; alternatively, a
hemostat can be used to clamp an elastic tube
or band wrapped around the animal’s leg. The
tourniquet or hemostat should be placed just
above the elbow and tightened firmly enough
to cause the vein to rise for easy visibility. A
tourniquet or hemostat can at times provide
better constriction of a vein than simply a
handler’s thumb; however, it can be dicult for
the technician to unclamp a mechanical device
once the needle has been inserted, so most
technicians prefer to rely on their partner for
manual hold-o.
Sodium Pentobarbital
Inserting needle into the cephalic vein
IV—Intravenous
(injection of sodium pentobarbital
into a vein)
Species recommended: dogs, calm
and friendly cats
Dose: 39 mg/pound (usually 1 ml/10 pounds)
Circulatory compromised: 78 mg/pound
(2 ml/10 pounds)
Injection speed: rapid (1-2 ml/second)
and consistent with good technique
Dog veins: cephalic, lateral saphenous
Cat veins: cephalic, medial saphenous
(femoral)
Syringe retracts: small volume (flash)
of blood
Time to loss of consciousness: ~5 seconds
Time to deep anesthesia: ~10 seconds
Time to cessation of respiration:
~20 seconds
Time to cessation of heartbeat
(death): ~40 seconds
Time to cardiac standstill (no fibrillation):
~2–5 minutes

14
The Humane Society of the United States Euthanasia Reference Manual
If the euthanasia technician is still having
trouble viewing the vein after applying su-
cient pressure to raise the vein, he or she can
“pump” the leg by holding the upper part of the
leg and moving the foot up and down several
times. This is somewhat like shaking a dog’s paw,
except that only the foot and carpus (the dog’s
“wrist,” or leg just above the foot) are moved.
Pumping the leg increases the return of blood
from the foot toward the heart and helps to
further increase the size and appearance of the
restricted vein. Rolling the leg slightly to one
side or the other can sometimes change the
visual angle enough to help the technician iden-
tify the vein. Finally, tapping the surface of the
forelimb with the index finger can also help con-
firm the presence of the vein, which should have
a more “springy” feeling than adjacent tissues.
Once the vein has been located and raised for
injection, the handler should continue to hold
o the leg (or leave the tourniquet in place)
until the euthanasia technician indicates that
the needle has been successfully inserted into
the vein and the injection of sodium pentobar-
bital is ready to proceed.
IMPORTANT NOTE: The euthanasia technician
must be able to see the vein, feel it, or both,
before inserting the needle into the animal’s
limb. It is not acceptable practice to “fish
around” for the vein (puncturing the skin then
moving the needle around in a blind attempt
to locate the vein), even if the technician is very
experienced and knows where the vein is most
likely to be located. While veins are generally
in the same place on every animal, slight ana-
tomical variations must be accounted for (for
example, some animals have veins that run
perfectly vertical in the center of the foreleg,
while others have veins that sit slightly o-cen-
ter, that bend slightly to one side or the other,
or that sit more or less deeply under the skin).
The technician should try to pierce the skin with
the needle only if he or she is confident that the
vein has been located and that there is a rea-
sonable expectation of piercing the vein on the
first try. If the technician has followed all of the
steps above to locate the vein without success,
he or she should either move on to another
leg vein for IV injection, switch to IP injection
if appropriate for the species and size of the
animal, or administer pre-euthanasia drugs to
render the animal unconscious so that sodium
pentobarbital can be administered IC.
Injection
The proper technique for injecting sodium
pentobarbital into a vein is not the same as
for administering vaccinations or other injec-
tions. The way the needle is directed, the angle
at which it enters the skin, even the way the
syringe is physically picked up and held are
dierent for administration of an IV injection,
and euthanasia technicians must know these dif-
ferences and be comfortable with them. When
administering a vaccination, the technician
typically holds the syringe between the index
and middle finger and places the thumb on
the plunger, so the hand eectively surrounds
the syringe. The orientation of the bevel (the
hole from which the liquid leaves the needle)
isn’t critically important when administering a
vaccination, nor is the exact angle of the injec-
tion. When administering IV injections, however,
care must be taken to ensure that the needle’s
Sodium Pentobarbital
Proper technique for picking up and
holding a syringe for IV injection

15
The Humane Society of the United States Euthanasia Reference Manual
bevel is pointed up (so the sharpest point is
the first to touch and enter the skin, ensuring
a clean puncture), and that the needle angle is
shallow enough to allow it to fully enter the vein
without piercing the far side. To achieve this, the
technician should pick up the syringe as though
it were glued to a table surface. Ideal finger
placement is for the thumb to sit on one side of
the syringe and for the first and second finger-
tips to sit on the other side, on the barrel away
from the needle end of the syringe (so there is
room for the technician’s other hand to properly
stabilize the needle, as discussed below), with no
part of the hand lying underneath the syringe.
This not only ensures that the syringe can have
fairly close contact with the skin of the animal,
but it also allows the technician to control the
needle angle most eectively.
Remember, even if your shelter already
has a policy in place to scan each stray
animal upon arrival for a possible micro-
chip or other identification (this should be
standard practice, and is legally required
in some states), each animal should be
scanned one final time before being euth-
anized. Animals too fractious or fearful to
be adequately scanned while conscious
can be scanned while under the influence
of pre-euthanasia drugs; if an identifica-
tion is found, the animal can be allowed
to awaken with no irreversible eects.
The hand not holding the syringe should
stabilize both the leg of the animal and the vein
itself. Ideally, the technician should wrap his
or her hand under the animal’s leg and cradle
the vein between the thumb and fingers; this
technique has the added benefit of creating a
kind of channel to help guide the needle into
the proper location in the center of the vein.
Alternatively, the technician can wrap his or her
fingers around the back of the animal’s leg and
lay the thumb flat along the vein from thumb-
nail to palm, applying gentle steady pressure so
that the vein does not roll away from the needle.
Once the handler has a firm but gentle grasp
on the animal, the vein has been definitively
located and stabilized, and the euthanasia
technician has the syringe at the ready (beveled
edge facing up), injection can begin. The first
injection attempt should be made into the vein
in the center of the shaved area (typically just
above the carpus, or wrist joint). The skin of
most dogs and cats is thin enough that it takes
only a small amount of pressure to make the
initial puncture; the technician will feel brief
resistance, then a smooth insertion under the
skin, although age, gender, and the medical
condition of the animal can make the skin a bit
more dicult to pierce.
One of the most dicult aspects of successful IV
injection is developing an appreciation for not
just the proper location for insertion but the
angle at which the needle should be inserted. IV
injection requires that the needle puncture the
skin and fully enter the vein so that the drug can
be injected directly into the bloodstream and
Sodium Pentobarbital
A syringe holding 6 ccs of euthanasia solution
Bevel
Needle
Hub
Barrel
Plunger

16
The Humane Society of the United States Euthanasia Reference Manual
carried swiftly to the heart (commonly referred
to as “hitting the vein”). If the initial approach is
at too shallow an angle, the needle may punc-
ture the skin but may not enter the vein, or the
needle may bounce o the top of the vein and
roll the vein away; in either case, if injection is
attempted, the drug will simply pool between
the skin and the vein. Alternatively, if the
approach is at too steep an angle, the needle is
likely to pass through the vein and out the other
side (given that most veins are just a few milli-
meters wide), again causing any drug injected
to miss the bloodstream. Generally, entering the
skin at a 30-degree angle is likely to produce the
greatest success; however, because all animals
are individuals there is no guaranteed perfect
angle, and the best way for a technician to maxi-
mize success is through practice and experience.
It can be dicult for the inexperienced tech-
nician to know when the needle has entered
the vein. Although not always present, there
is frequently a “flash” of bright red blood that
appears in the hub of the needle (the plastic part
of the needle that attaches to the syringe) when
the vein is pierced. This may be accompanied
by a very slight popping feeling as the needle
pierces the vein wall and enters the bloodstream,
after which it should meet little resistance unless
it is inserted so far that it pierces the other side.
Feeling the vein is one of the most dicult skills
for the new euthanasia technician, but with
patience and practice it can be readily mastered.
Once the vein has been entered, the techni-
cian must continue to insert the needle until a
sucient portion of its length is inside the vein.
If only the tip or a very small portion of the nee-
dle is inserted, the needle is likely to be knocked
out of the vein once pressure is applied to the
plunger to inject the drug. However, care must
be taken to ensure that the needle is not thrust
so far in that it pierces the vein’s far wall. Again,
practice and experience are the best teachers.
Because proper placement of the needle inside
the vein is so critical, once it has been achieved
the technician must secure the syringe in place
before any drug is injected. This is important
not just because the pressure of injection could
cause the needle to move, but also because the
animal may at any point jerk his or her leg away,
altering placement of the needle. If the syringe
is clamped securely to the leg, however, the tech-
nician can usually continue the injection even
though the leg is moving or wait a few seconds
until movement has stopped and then continue.
Sodium Pentobarbital
Because there are nerve endings in the
skin, animals may react to the initial
needle puncture. Ensuring that the needle
is new and that the animal is securely but
gently restrained can help minimize the
impacts of such a reaction. If the animal
reacts slightly, an experienced technician
can continue the process by remaining
calm and moving quickly through the next
steps; in most cases the animal will mirror
the technician’s calm demeanor and settle
back down. If the animal does not calm
down, however, and it becomes unsafe to
proceed, the procedure should be halted,
the needle removed, and the injection site
should be covered with light pressure to
stop the leakage of blood. In such cases it
will be appropriate to use a pre-euthana-
sia drug to minimize further distress.
After insertion, the technician should use
his thumb to secure the needle

17
The Humane Society of the United States Euthanasia Reference Manual
Typically, the technician secures the syringe by
taking the thumb that was formerly supporting
the vein for initial injection and clamping it down
over the syringe, securing it firmly between the
thumb and the animal’s leg. Thumb placement is
important—it must be back far enough to secure
the body of the syringe, rather than the needle,
and it must not block the technician’s view of
the hub (it is necessary for the technician to look
for blood in the hub when the needle is aspi-
rated). Alternatively, the technician may secure
the syringe by clamping it between the thumb
and fingers of the hand holding the leg. At first
these movements may seem awkward, because
they mean loosening the grip on the animal’s leg
momentarily and finding just the right spot to
hold the syringe securely in place. With practice,
however, it becomes a quick, smooth motion
that secures the syringe firmly without any shift
in needle location.
Injecting into veins is a skill that requires
experience to master; fortunately, new
euthanasia technicians have ways to
practice without inflicting suering on an
animal. Inexperienced technicians can use
an orange to simulate the feeling of push-
ing a needle through skin. Alternatively,
they can practice on animals that have
already been euthanized. While this may
sound inappropriate, it is better that tech-
nicians develop their skills using animals
that can no longer feel pain than live ani-
mals. While being technically proficient
at euthanasia is a skill no one expects or
desires to have to master, if euthanasia
must be performed it is best done by
someone who is as skilled as possible.
Verifying that the Needle Is in the Vein
(Aspirating Blood)
Once the syringe has been secured to the leg,
the technician must confirm that the needle
is properly in the vein. To do this, he or she
attempts to aspirate (draw back) blood into
the syringe. When the plunger of a syringe is
pulled back, it sucks in whatever material the
needle is exposed to—if the needle is immersed
in water, for example, pulling back the plunger
(aspirating) will draw water into the syringe.
Likewise, if the needle is immersed in blood, as
it should be if it is properly inserted into a vein,
pulling back on the plunger will draw blood
into the syringe. This is the most definitive sign
that the needle is properly placed for injection
of sodium pentobarbital. If pulling back on the
plunger produces either no blood at all or just
a slight flash of blood, the needle is not actually
in the vein and it must be repositioned before
any drug is injected. IMPORTANT NOTE: When
aspirating, pull back on the plunger gently;
pulling back too aggressively can draw the wall
of the vein into the needle tip (“collapsing” the
vein), blocking the needle.
To reposition the needle, it is often not nec-
essary to remove it fully from underneath the
skin. In fact, it is preferable to simply reposition
the needle under the skin to avoid causing
the animal pain by reinjection. The technician
should use his or her senses to determine
whether the needle is sitting above, below, to
the right, or to the left of the vein and adjust
position accordingly. Once the needle has been
repositioned, it should once again be secured
and aspiration attempted. It may be necessary
to move the needle several times before suc-
cessful aspiration of blood confirms that it has
been properly placed; this happens to even the
best and most experienced technicians. The
important thing is not to become frustrated or
Sodium Pentobarbital
It is a commonly held misconception that
second injections must always be per-
formed above the initial injection site; in
actuality, the structure of veins is such that
they will eectively carry blood to the
heart even if they encounter a puncture.

18
The Humane Society of the United States Euthanasia Reference Manual
upset, since humans’ moods can easily be read
by the animal—simply take a breath, regroup,
and try again.
IMPORTANT NOTE: If it appears from all
indications that the needle is actually in the
vein but nevertheless no blood can be aspi-
rated, the needle may be clogged. Dirt from
the animal’s skin, skin tissue, blood from a
previous insertion attempt, or a hair particle
may be caught in the shaft, blocking delivery
of the drug. The only way to confirm this is to
withdraw the needle, aim it away from any
animals or people, and gently attempt to push
a small amount of solution out of the syringe.
If nothing comes out, the needle is clogged
and should be discarded. Use a new needle to
attempt another injection.
If it is necessary to remove the needle from the
leg, remove the pressure being used to raise
the vein (loosen the tourniquet or release the
hold on the vein) and apply gentle pressure to
the puncture site to try to avoid the formation
of a hematoma or swelling created by blood
leaking from the pierced vein.
The second injection attempt should be made
either slightly above or slightly below the
initial puncture site. If the animal begins to
resist, let him or her relax for a moment by
loosening (but not releasing) the restraint.
IMPORTANT NOTE: Every time you pierce the
skin the needle becomes dull, and can cause
the animal pain. If the animal is conscious and
you have missed the vein, at a minimum you
should replace the needle; a better practice is
to simply abandon the attempt to use direct
injection and opt instead to anesthetize the
animal before attempting further injection.
If the animal is unconscious and the vein
continues to be missed, the technician can
simply move on to another leg. If the techni-
cian is still not having success in hitting the
vein, consider passing the syringe to another
trained technician. Every euthanasia technician
has experienced being seemingly completely
unable to hit a vein, only to have her partner
hit the vein on the first try; while understand-
ably frustrating, it is important to shake this
o and move on with the work, since everyone
has a bad day from time to time. One final tip
on hitting veins: Those just learning the art of
intravenous injection tend to be very cautious,
and rightly so. Their tendency is to hesitate
and advance the needle very slowly; however,
a slow-moving needle is more apt to push the
vein o to one side, rather than pierce it, and
piercing the skin slowly is more painful than
moving through the skin deftly. Practice will
help the inexperienced technician become
more confident and slide the needle into the
vein with a deliberate, yet gentle motion.
Injecting the Drug
Once blood is clearly aspirated into the syringe,
allowing the euthanasia technician to confirm
that the needle is properly located in the vein,
the sodium pentobarbital can be injected. The
technician should signal the handler to remove
the pressure from the vein (or release the tour-
niquet) so that full blood flow to the heart is
restored; if injection is attempted before pres-
sure is released, the leg will begin to swell as
a result of the backup.
Sodium Pentobarbital
Injection into the cephalic vein of an unconscious dog

19
The Humane Society of the United States Euthanasia Reference Manual
When administering vaccinations or injecting
pre-euthanasia drugs, best practice is to inject
the drug slowly, so as to limit stinging; by
contrast, sodium pentobarbital should generally
be administered quickly, so that the maximum
amount of drug possible is carried to the heart
and then the brain at one time (this eect of
getting a large quantity of drug to reach the tar-
get at one time is called a bolus eect). Injecting
the drug too slowly may cause the animal to
move slowly between Stages I and IV and have
adverse reactions (howling, uncoordinated
movement, anxiousness, etc.) before losing
consciousness. IMPORTANT NOTE: Drugs that
combine sodium pentobarbital with phenytoin
sodium should be administered slowly, since the
presence of phenytoin sodium is sucient to
avoid the need for bolus administration.
Nevertheless, whenever injecting drugs, make
certain that the pressure on the syringe is not
so great that the needle either moves out of the
vein or pops o the end of the syringe. When
the injection is performed correctly, the animal
should begin to lose consciousness and collapse
three to five seconds after the first milliliter of
sodium pentobarbital is introduced into the
vein; the technician should continue to inject
the sodium pentobarbital until the syringe is
empty, and the handler should continue to gen-
tly restrain the animal until he or she has fully
lost consciousness. If the injection is successful
there will be no visible change to the leg during
or after injection; if, however, a bulge appears,
the vein wall has been compromised, drug is
escaping and injection should cease immedi-
ately (this is commonly referred to as having
“blown” the vein).
Removing the Needle
If the animal was conscious when direct IV
injection was performed, he or she will typi-
cally lose consciousness and begin to collapse
before the injection is completed. The handler
should take care to gently lower the animal to
the table or floor once he is unable to stand on
his own, ensuring that the syringe remains in
place until fully withdrawn by the technician,
in addition to showing respect for the animal.
When the animal is completely unconscious
and his body is resting fully on the table or
floor, the technician may remove the needle
from the vein by withdrawing it slowly; slight
pressure should be placed on the injection
site to prevent blood from seeping from the
puncture. IMPORTANT NOTE: Verifying death
(see Chapter 4) is a required next step. The
euthanasia process is never finished until death
has been verified.
Special Considerations for Injecting
into the Saphenous Vein
The procedure for injecting into the saphe-
nous vein is generally the same as that for the
cephalic vein, except for how the vein is held
o and how the needle is inserted.
Holding O the Saphenous Vein
To hold o the lateral saphenous vein, the
handler usually places his hand around the leg
just above the stifle (knee), so that the thumb
is on the back side of the leg and the fingers
on the front surface of the leg. The vein is then
held o by gently but firmly squeezing the leg
Sodium Pentobarbital
Light pressure on the puncture after
injection will prevent bleeding

20
The Humane Society of the United States Euthanasia Reference Manual
and the vein between the thumb and fingers;
when properly performed, this pressure will
cause the leg to extend. The primary goal is
to get sucient pressure over the saphenous
vein as it runs down the rear leg to both raise
it and stabilize it for injection. For the medial
saphenous vein, the handler may either hold
o in a similar fashion, only the leg is reversed,
or may apply pressure with the outer edge of
the palm, usually while holding the animal’s
leg or tail. IMPORTANT NOTE: The closer the
technician gets to the animal’s joint the higher
the likelihood that the vein will roll; injecting
higher up and away from the joint proves
much easier. A commercial tourniquet, how-
ever, does not hold its position well when used
above the joint, so a partner able to manually
hold o the saphenous vein is necessary.
Injecting the Drug
Because the saphenous vein is much freer to
move under the skin than the cephalic vein,
the needle must be inserted with a quick
puncture, threading into the vein quickly and
stabilizing its movement. If the technician
tries to place the needle over the vein and
slowly insert it, particularly if the injection site
is too close to the joint, the saphenous vein
will roll out from under the needle and o to
the side. When injecting into the saphenous
vein it is important for the handler and tech-
nician to communicate eectively, since the
more pressure that is applied to stabilize the
vein, the more dicult it can be for the tech-
nician to feel.
Once the needle is in the vein, the same proce-
dure should be carried out as for injection into
the cephalic vein. The syringe must be secured
in place and aspirated, the pressure on the vein
released, and the drug injected. NOTE:
Securing the syringe to the leg may be slightly
more awkward than with the cephalic vein, as
the syringe and rear leg will not be aligned in
exactly the same direction.
Special Considerations for
Injecting into the Jugular Vein
As noted above, while it is technically possible
to use the jugular vein for euthanasia of cats
and dogs, it is not recommended. However,
for livestock, equines, and other large mam-
mals, the jugular veins are typically preferred
because they are large and readily accessible,
they are capable of handling the large vol-
umes of sodium pentobarbital necessary,
and because trying to inject into the leg of a
conscious equine or other large animal poses
grave safety risks for the technician and han-
dlers. IMPORTANT NOTE: Large animals can
be dangerous and pose special risks even after
injection, so even technicians with extensive
experience with small animals should defer
to the expertise of a large-animal veterinar-
ian. (See Chapter 12 for more information on
euthanasia of large animals.)
Holding O the Jugular Vein
To hold o the jugular vein, one or more fingers
should be pressed against the vein below the
injection site in the jugular furrow (blood in
the jugular vein runs down the neck toward
the animal’s heart). Since a tourniquet or other
mechanical means cannot be used to hold o
the jugular vein, the technician must become
proficient at holding o the vein herself, plac-
ing the fingertips flat across the vein to provide
the maximum area of pressure.
Sodium Pentobarbital
Holding a cat for injection into the medial saphenous vein
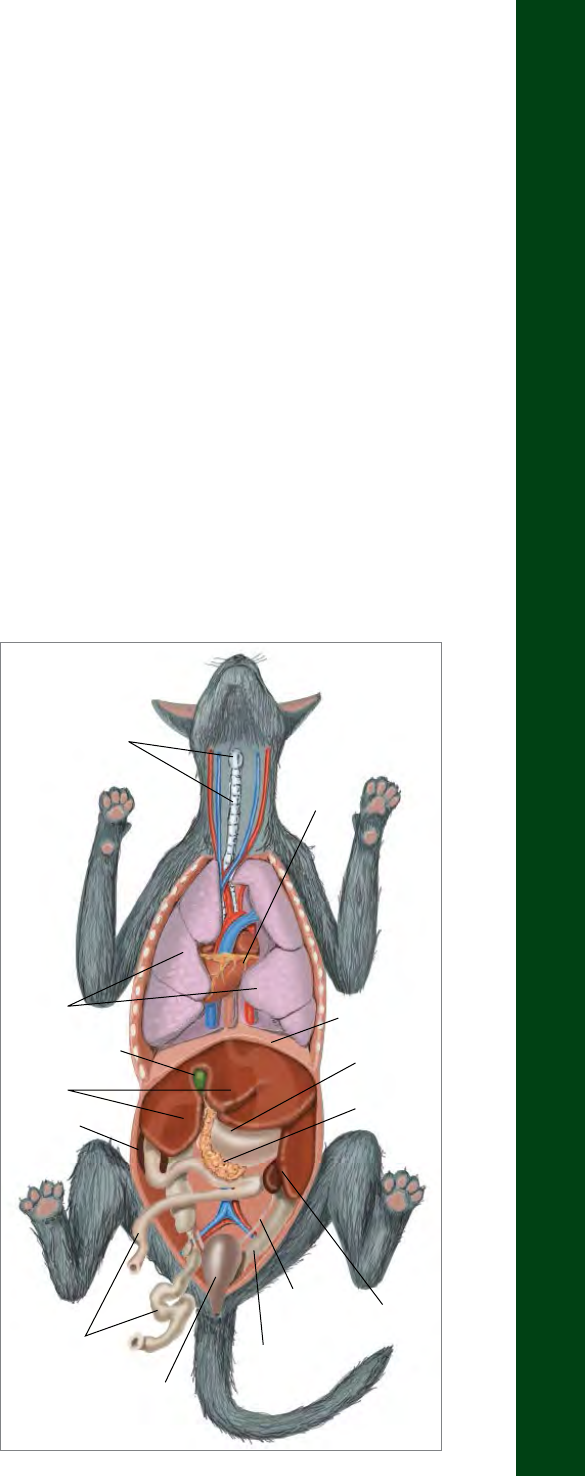
21
The Humane Society of the United States Euthanasia Reference Manual
Problems with IV Injection
(Commonly Referred to as
‘Blowing the Vein’)
Proper IV injection involves inserting the needle
inside the vein and ensuring that 100 percent
of the drug injected enters the bloodstream
and flows to the heart. If the needle pierces
the vein wall (either because the initial needle
insertion was imprecise or because movement
by the animal or technician pushed the needle
out of the vein) or misses the vein entirely, and
drug ends up injected outside the normal flow
of blood, the drug will pool under the skin and
make the area swell up (this is clinically referred
to as “extravascular injection", injection of drug
outside the vein). This is what euthanasia tech-
nicians commonly refer to as “blowing the vein,”
and it can happen to even the most skilled tech-
nicians. When it occurs, the technician should
not panic but should take steps to correct the
problem and ensure that the euthanasia process
continues as quickly and humanely as possible.
Technically speaking, if sodium pentobarbital
is injected outside the vein, the animal will
experience pain because the pH level of the
drug is higher than that of blood, and because
the tissue surrounding the veins is rich in nerve
endings (unlike the walls of the veins them-
selves). In practice, however, unless the vein was
missed entirely, it most often happens that some
of the sodium pentobarbital does reach the
brain and quickly renders the animal insensitive
to pain, allowing the technician time to regroup
and reinject the drug properly. Nevertheless,
the possibility of causing pain to an animal
through improper injection exists, and techni-
cians must recognize that an animal in pain can
pose a significant danger to himself or herself
and to humans; this should serve as a sobering
reminder that: a) technicians must never inject
blindly, without some indication that the needle
is actually in the vein; b) technicians must be
careful to secure the syringe and not allow the
needle to move once it is properly inside the
vein; and c) handlers must ensure each animal is
adequately and humanely restrained through-
out the entire injection process.
If the technician believes that the vein has been
blown (as evidenced by swelling indicating
that the drug is outside the vein or by a pain
reaction from the animal), he or she should stop
injecting immediately, withdraw the needle,
and apply pressure to the injection site to stop
the flow of blood—there is no point in continu-
ing to inject additional drug, since it will not
be properly carried by the bloodstream to the
heart. If the animal is still calm and tractable, or
if the animal is unconscious, the technician can
simply attempt a second injection. However, if
the animal is either reacting in pain or becomes
caught in the excitement phase of anesthesia,
Sodium Pentobarbital
View of a cat’s internal organs, as seen from above
Larynx &
Trachea
Lungs
Gallbladder
Liver
Kidney
Small
Intestines
Urinary
Bladder
Large
Intestines
Uterus &
Ovaries
Spleen
Pancreas
Stomach
Diaphragm
Heart

22
The Humane Society of the United States Euthanasia Reference Manual
it is neither safe nor practical to continue
trying to administer sodium pentobarbital by
IV injection—the animal will not be suciently
manageable to safely administer the drug. In
such cases, a pre-euthanasia drug should quickly
be administered into the large muscle mass of
the leg or back so that the animal is rendered
unconscious as quickly as possible and humane
injection of sodium pentobarbital can proceed.
IV Dosage of Sodium Pentobarbital
For IV injections of dogs and cats, regardless of
the vein used, the standard dosage of sodium
pentobarbital in commercial formulations like
Fatal-Plus and Pentasol is 1 milliliter for every
10 pounds of body weight. IMPORTANT NOTE:
The commercial formulation of sodium pento-
barbital known as Sleepaway requires dierent
dosing, so refer to the product label for details.
For the purposes of this manual, sodium pento-
barbital dosage information will refer to other
commercial formulations like Fatal-Plus. That
means for IV injection, a cat weighing 10 pounds
should receive 1 milliliter of sodium pentobar-
bital, and a 100-pound dog should receive 10
milliliters. A simple method to calculate the
appropriate IV dosage of sodium pentobar-
bital is to divide the animal’s body weight (in
pounds) by 10, which equals the volume of drug
in milliliters to be administered. Technicians
can help minimize the chances of record-keep-
ing errors by rounding up (never down!) to
the nearest milliliter; entering fractions into a
euthanasia logbook (see Chapter 10 for infor-
mation on record-keeping requirements for
drugs like sodium pentobarbital) is an invitation
for mathematical errors. Remember, when
using the label dose, the technician is admin-
istering more than the lethal dose; rounding
up and administering slightly more drug than
technically needed is perfectly acceptable (it is
never acceptable to use less than the label dose).
Therefore, for an 18-pound animal, even though
the accurate label dose is 1.8 milliliters, adminis-
tration of 2 milliliters is appropriate.
Intraperitoneal (IP) Injection
(Injection of Sodium Pentobarbital
into the Abdominal Cavity)
Intraperitoneal (IP) injection involves the
injection of sodium pentobarbital directly into
the animal’s abdominal cavity, the space in the
abdomen surrounding most of the internal
organs. While at first glance this method might
appear to be painful, and therefore inappropri-
ate for euthanasia, when performed properly
it is completely painless for the animal and can
be more humane than even IV injection in some
circumstances. With IP injection, the needle is
inserted into the narrow band of connective
tissue that runs vertically along the center of
the abdomen called the linea alba (imagine the
center line of the “six pack” on a person with
well defined abdominal muscles); because there
are no nerve endings or blood vessels along this
line, there is no pain or sensitivity upon inser-
tion of the needle or during injection. IP is the
most humane option for euthanasia of con-
scious cats because it requires even less restraint
than IV injection; cats tend to resist extending
a forelimb for IV injection, but they are often
very amenable to the gentle lifting or cuddling
Sodium Pentobarbital
In the past, technicians were advised
to engage in a practice called “dosing
for eect,” adding an extra one to two
milliliters of drug to compensate for
circumstances like pregnancy, heavy
musculature, and others. Current practice
is to “dose for eect” only those animals
suering from circulatory problems
(as evidenced by severe dehydration,
external bleeding or pale gums), giving
them twice the normal dosage of drug
recommended. Extra drug may also be
administered to an animal whose owner
is present, not because it is technically
required but to ensure that death is
achieved as quickly as possible.

23
The Humane Society of the United States Euthanasia Reference Manual
motion associated with IP injection. IP is also a
good option for kittens, puppies, birds, reptiles,
small mammals, and small wildlife, whose veins
are dicult to locate. IP is not, however, a good
option for late-term pregnant animals, or for
adult dogs and puppies over five weeks of age
because they are more likely to experience a
panic/excitement response, and because IP
requires the injection of three times the quan-
tity of drug that IV does.
When sodium pentobarbital is injected into
the abdominal cavity, it begins to disperse over
the large surface area of the intestines and
internal organs. After several minutes, the drug
starts being absorbed into the bloodstream
and slowly travels to the brain. This process
of absorption makes IP a slower process than
IV, where the drug is injected directly into the
bloodstream. The time period between injec-
tion and death is typically less than 10 minutes,
and it is vital that during this time the animal
be placed in a dark, quiet, confined area (like a
carrier covered with a thick towel or blanket)
while the drug takes eect.
While the slower time to take eect and triple
dosages can be considered disadvantages
of IP administration, they must be weighed
against the stress that would be caused to the
animal if he or she were restrained for an IV
injection and the inherent diculties trying
to insert a needle into very tiny veins. For
Sodium Pentobarbital
Units of Measure
cc = cubic centimeter (measure
of volume/space)
ml = milliliter; one 1000th of a liter
(measure of liquid volume)
mg = milligram; one 1000th of a gram
(measure of weight/mass)
1 cc is generally equivalent to 1 ml
Intraperitoneal (IP)
(injection of drug into the abdominal
or peritoneal cavity, the gap between
organs and the abdominal wall)
Primary Advantages
• Can be used with the very youngest
or smallest of mammals, where finding
a vein is dicult.
• Often preferred for use on conscious,
well-socialized adult cats because it
does not require invasive handling.
• Restraint of the animal for IP injection
does not put the euthanasia technician
in close proximity to the animal’s teeth
or claws as restraint for IV injection.
• Virtually pain-free for the animal when
performed correctly.
Primary Disadvantages
• Improper injection can result in drugs
introduced into fat or organs, making
absorption less eective and potentially
causing pain to the animal.
• Takes a longer time before unconscious-
ness and death, because the drug must
first be absorbed into the bloodstream
before being carried to the heart
and brain.
• The animal must be held in a safe, quiet,
secure area after drug administration
until it fully loses consciousness.
• Requires a much larger volume of
sodium pentobarbital than other
methods (three times more drug).
• Can be perceived as painful by those
who misunderstand the technique (an
untrained individual may not understand
that there are no nerve endings along
the linea alba, for example, and may inac-
curately assume that IP involves a painful
injection into the animal’s stomach).
• Should not be used on adult dogs because
of an increased chance that they will expe-
rience a prolonged excitement phase.

24
The Humane Society of the United States Euthanasia Reference Manual
well-socialized cats, kittens, and other small
mammals, IP can in many cases be the more
humane and eective method of administering
sodium pentobarbital.
Administering IP Injection
To administer an IP injection, the animal must
be gently lifted so that its abdomen is acces-
sible to the euthanasia technician. This can be
achieved with a well-socialized adult cat, for
example, by simply gathering the cat’s front
legs and lifting them slightly o the exam table;
the drug can then be injected without the cat
even realizing the procedure is happening.
Once the belly is accessible, injection should
be made into the animal’s ventral midline. The
ventral midline injection site is midway down
the abdomen, just below the umbilicus (belly
button). The xiphoid, a cartilaginous protrusion
at the base of the sternum (the breast bone),
makes for a good landmark in locating this
injection site. Look for the xiphoid, and draw
an imaginary vertical line down the animal’s
abdomen from that point (remember, there are
no nerve endings or blood vessels along this
imaginary vertical line, called the linea alba).
Next, look for the animal’s hip joints, and draw
an imaginary horizontal line between them. The
intersection of the imaginary horizontal and
vertical lines will indicate the point on the ani-
mal’s midline just below the belly button where
it is safe to insert the needle for an IP injection.
When the euthanasia technician successfully
injects into the ventral midline the drug will be
properly injected to the peritoneal cavity, and
the injection will be completely painless.
While injecting into the midline does not require
quite as much precision as injecting into a vein,
it is still critical to properly locate the injection
site. Injection into the left side of the abdomen
should be avoided because of the possibility
of injecting into the spleen, which could delay
absorption of the drug. The lower part of the
abdomen should be avoided because if the
drugs are injected into the bladder they may
simply be excreted through the urine before
being absorbed. Injection into the liver (techni-
cally intrahepatic injection) should be avoided as
well, as there remain questions about the liver’s
sensitivity to pain. Finally, and perhaps most
important, injection into any other area of the
abdomen aside from the ventral midline will be
painful for the animal, and must be avoided.
It is not necessary to shave the abdominal area
before IP injection; simply insert the needle
straight into the ventral midline of the abdomen
Sodium Pentobarbital
Proper hold and injection site for IP injection of a cat
It is sometimes recommended that IP
administration be achieved by grasping
the animal’s front and rear legs and
stretching the body to expose the abdo-
men. Stretching, however, is not only
uncomfortable for most animals, but it
can also result in increased pain sensation,
as the stretched skin can be more sensitive
to pain. If IP cannot be achieved by simply
gently lifting the forelimbs of the animal,
an alternate method of administration
should be considered.

25
The Humane Society of the United States Euthanasia Reference Manual
at a 90-degree angle to the body (as opposed
to the much shallower angle used when inject-
ing into a vein). The smallest gauge needle
available is recommended; typically a 22-, 23-,
or 25-gauge, three-quarter-inch to one-inch
needle is used. Upon aspirating (pulling back)
the syringe’s plunger, a vacuum with no blood
or other bodily fluid entering the syringe should
be observed, indicating that the needle has
been successfully inserted into the abdominal
cavity; if there is fluid or other material in the
syringe the needle should be withdrawn and
repositioned, because it may be mistakenly
located in the spleen, bladder, or other organ. If
the needle appears to be located properly, mean-
ing no blood, urine, or other bodily fluid appears,
inject the drug at the steady rate of 1 milliliter per
second. IMPORTANT NOTE: While the peritoneal
cavity is normally empty, creating a vacuum
on aspiration, there are situations in which the
needle is properly in the cavity but fluids are
nevertheless aspirated. Blood may be present as
a result of internal injuries, or feline infectious
peritonitis or other diseases could cause pus to
exist there. An experienced euthanasia technician
will be able to tell whether fluids aspirated into
the syringe indicate that the needle has been
improperly inserted or fluids have invaded the
cavity; if in doubt, they should either reposition
the needle and try again, or opt for an alternate
method of administering sodium pentobarbital.
Because IP does not induce immediate uncon-
sciousness, all animals given an IP injection
should be immediately placed in a cage or ken-
nel with soft bedding where they can remain
undisturbed until they have lost consciousness.
The cage or kennel should be covered with
a towel or blanket, or the lights in the room
should be dimmed, and any noise minimized to
avoid any undue stimulation of the animal as
the drug takes eect. Although most animals
should be placed alone in a cage or kennel after
IP injection, littermates can be placed together
to provide them more comfort and security.
IMPORTANT NOTE: If a mother and litter are
to be euthanized, the mother should be eutha-
nized before the litter to help reduce her stress
to the greatest extent possible.
Sodium Pentobarbital
Because of the indirect absorption of the
drug when IP is used, animals may receive
enough drug to enter the involuntary
excitement phase of anesthesia but not
enough to move toward complete uncon-
sciousness and death. Handling of animals
in this state should be avoided.
IP—Intraperitoneal
(injection of sodium pentobarbital into the
abdominal cavity—needle passes through
the skin, muscle wall and peritoneum)
Species recommended: conscious calm
and friendly cats, conscious puppies under 5
weeks, conscious or unconscious small rodents
Dose: 117 mg/pound (usually 3 ml/
10 pounds)
Circulatory compromised: not usually
recommended
Injection speed: slow (0.5–1 ml /second)
and consistent with good technique
Needle insertion point: ventral, midline,
~2" below (caudal to) umbilicus
Needle angle/depth: right angle to
the skin/~3/4 inch
Syringe retracts: suction (no fluid, no air)
Time to loss of consciousness: ~2 minutes
Time to deep anesthesia: 3–4 minutes
Time to cessation of respiration: ~6 minutes
Time to cessation of heartbeat (death):
~8 minutes
Time to cardiac standstill (no fibrillation):
~10 minutes

26
The Humane Society of the United States Euthanasia Reference Manual
As an animal passes through the early stages of
anesthesia after IP injection, several signs may
be noted. One of the first is a characteristic
tongue-licking, typically seen about two
minutes after IP injection. Thereafter, the
animal typically loses coordination and may fall
over or begin to flail—the animal is not experi-
encing pain at this point, but is still capable of
reflexively biting if startled, making this a
potentially dangerous time for a euthanasia
technician or handler. Minimizing environmen-
tal stimuli, like noise and light, is especially
important during this stage. If the animal has
not begun to show signs of moving through
the stages of anesthesia toward unconscious-
ness and death within 10 minutes after IP
injection, it is possible that the drug was
mistakenly injected into (and subsequently
expelled from) the bladder, or that its absorp-
tion has been otherwise impeded, usually by
absorption into fat. The animal should be
injected again with additional drugs, either by
IP or IV injection. Even after the animal appears
to be unconscious (lying still, no blink or toe
reflexes), it may take 10 to 15 minutes more for
clinical death to occur. IMPORTANT NOTE:
Never assume that an unconscious animal is
dead! Always take proactive steps to verify
death (see Chapter 4).
IP Dosage of Sodium Pentobarbital
The standard dosage for IP injections (in
commercial formulations like Fatal-Plus and
Pentasol) is 3 milliliters for every 10 pounds
of body weight, or triple the dosage used for
an IV injection (the larger dose is required
because the drug must be absorbed into the
bloodstream before beginning its trip to the
brain). A minimum dosage of 1 milliliter of
sodium pentobarbital should be used on any
animal under 3 pounds (except neonates and
very small animals like young mice, etc., whose
bodies may not be large enough to absorb a
full milliliter of drug.)
Intracardiac (IC) Injection
(Injection of Sodium Pentobarbital Directly
into the Heart)
An intracardiac (IC) injection involves the
injection of sodium pentobarbital directly into
the heart, where it is quickly transported to the
brain. Injection into a conscious animal’s heart
is excruciatingly painful, even if the technician
is able to locate the heart chamber on the first
attempt. For this reason, IC injection must
never be administered to an animal unless
Sodium Pentobarbital
Intracardiac (IC)
(injection of drug directly into a chamber
of the heart)
Primary Advantages
• Ensures the fastest delivery of drugs
of any method (because the drug
has a direct route from the heart
to the brain).
Primary Disadvantages
• Tremendously painful for conscious
animals, so it must never be used unless
the technician has confirmed that the
animal is fully unconscious.
• Aesthetically unpleasant to observe
(therefore not appropriate for cases
where the owner or a member
of the public is present, even if
the animal is unconscious), and
often misunderstood by members
of the public.
The most common impediment to IP
administration is fat—if sodium pentobar-
bital is inadvertently injected into fat, the
drug will not readily move through the
circulatory system to the brain. Therefore,
an obese animal should not be considered
a candidate for IP injection.

27
The Humane Society of the United States Euthanasia Reference Manual
the euthanasia technician has confirmed that
the animal is fully unconscious. Many states
and municipalities have laws dictating that
animals must be fully unconscious before an
IC injection. Assuming that the technician has
ensured that the animal is unconscious, though,
IC can be the most ecient method of admin-
istering sodium pentobarbital, particularly if:
a) the animal’s veins have been compromised
because of illness or injury; b) the animal’s circu-
latory system is too compromised to transport
the drug from a vein to the brain; or c) sodium
pentobarbital has already been administered
to the animal through IV or IP injection but has
not eectively resulted in death.
Administering IC Injection
First and foremost, the technician must always
verify that the animal is fully unconscious
before attempting IC injection (this could be
because of illness or injury, or it can be induced
via a pre-euthanasia drug). An animal can be
considered fully unconscious if she has neither
a palpebral (blink) reflex nor a toe-pinch reflex.
To check for a blink reflex, the technician
should gently touch the inside corner of the
eyelid—if the animal does not reflexively blink,
she can safely be concluded to be unconscious.
To check for a toe-pinch reflex, which assesses
whether the animal is still able to withdraw
from deep pain, the technician should pinch
firmly on the soft skin (or webbing) between
the toes of a rear leg; if the animal does not
respond by reflexively pulling her leg back and
away from the pain, it is unconscious, and IC
injection can be administered. If the animal
still has either a blink or a toe-pinch reflex,
either give the animal more time for the drugs
to take eect or administer additional pre-eu-
thanasia drugs.
Sodium Pentobarbital
Anatomy of the heart
Aorta
Left atrium
Left atrioventricular valve
Aortic valve
Pulmonary valve
Left ventricle
Myocardium
Pericardium
Right ventricle
Arterial blood
Venous blood
Caudal vena cava
Right atrioventricular valve
Right atrium
Cranial vena cava
Body
Head Lungs

28
The Humane Society of the United States Euthanasia Reference Manual
Once the animal is confirmed to be uncon-
scious, IC injection can proceed; however,
locating the chambers of the heart for proper
IC injection is not always easy, even for an
experienced euthanasia technician. On most
unconscious animals that are still breathing
and circulating blood, the heartbeat can be felt
on the skin surface using the fingers, or heard
with a stethoscope. Injection should be made at
the point where the heart is heard or felt most
prominently, although the exact position will
vary according to the animal’s species, age, size,
and weight. If the heart cannot be easily heard
or felt, a good reference point for the correct
injection site is the point where the animal’s
elbow aligns with the body: while the animal is
lying on his or her side, bend the front leg back
slightly toward the rib cage so that the shoulder
is at a 45-degree angle; the proper injection site
should be directly behind the animal’s elbow.
Alternatively, the euthanasia technician can
count the animal’s ribs and inject into the lower
side in the third, fourth, or fifth, “intercostal”
spaces (the spaces between the ribs). The best
technique for identifying the proper location
is to practice on a live dog—as the dog is lying
down, count back the spaces between the
ribs beginning at his front leg—you will feel
a strong heartbeat in the area of the third,
fourth, and fifth spaces.
Because the heart is much farther below the
skin surface than a vein, it is typically necessary
to use a much longer needle for IC injection
than is used for IV or IP injections. A needle
as long as two inches may be required for
obese or deep-chested animals (the techni-
cian need not worry about pain a large-bore
needle may cause because the animal is con-
firmed unconscious and unable to feel pain).
NOTE: Clipping or shaving is not necessary
for IC injections because nothing on the skin
indicates the heart’s location.
When performing an IC injection, the needle
should be inserted straight into the chest
cavity at a 90-degree angle, as opposed to
the much shallower angle required for IV
injection, and the technician should ensure
that the needle is inserted between ribs (if
the needle hits a rib the technician will feel
resistance, and should reposition). The tech-
nician should then aspirate (pull back on the
Sodium Pentobarbital
There are few technical aspects of eutha-
nasia that generate more intense debate
than IC injection. Stories of conscious
animals being injected IC horrify the
public, and rightly so. However, when
properly administered on an unconscious
animal, IC can be a very eective means of
achieving a quick, painless death. Shelters
can navigate this potential publicity mine-
field by assuring the public that their sta
is properly trained, their policies reflect
the highest standards of humane care,
that “heart sticks” are never performed
on conscious animals under any circum-
stances, and that checks and balances are
in place to ensure that humane protocols
are always followed.
Checking for absence of blink reflex

29
The Humane Society of the United States Euthanasia Reference Manual
plunger) to ensure that the needle is in one
of the chambers of the heart, rather than in
the muscle of the heart or elsewhere. If the
needle has been properly inserted into
a chamber of the heart, a rapid, strong
flow of blood will be observed when the
plunger is pulled back, and the syringe will
rapidly fill with blood. If blood is not easily
aspirated, or if only a quick flash of bright red
blood is observed, the needle may be in the
heart muscle, or may have missed the heart
entirely; the needle should be redirected until
a strong flow of blood is easily aspirated,
indicating it is correctly located in a heart
chamber. IMPORTANT NOTE: Injection into
the heart muscle, rather than a chamber, is
not considered to be proper IC injection; that
is actually intramuscular (IM) injection, which
is not considered to be an acceptable route
of administration of sodium pentobarbital.
Therefore, just watching for movement of
the needle is not sucient, since injection
into the heart muscle will cause the needle
to move; the technician must observe the
strong, rapid flow of blood into the needle.
Once the technician has verified proper
insertion, the sodium pentobarbital can be
injected. After an IC injection is completed,
the needle should be left in place until the
heart has been observed to stop beating to
facilitate verification of death (see Chapter 4).
It generally takes seconds for death to occur
(similar to the time frame for an IV injection).
IC Dosage of Sodium Pentobarbital
The correct dosage for IC injections is the
same as for IV injections, 1 milliliter for every
10 pounds of body weight (in commercial
formulations like Fatal-Plus and Pentasol).
Other Injection Routes—
Not Acceptable
Aside from IV, IP, and IC, no other injection
method for sodium pentobarbital is acceptable
(this includes intramuscular, subcutaneous,
intrathoracic, intrapulmonary, intrahepatic,
intrarenal, intrasplenic, intrathecal, or any
other nonvascular injection site).
Sodium Pentobarbital
IC—Intracardiac
(injection of sodium pentobarbital into
one of the four heart chambers)
Unconscious animals only
Species recommended: all
Dose: 39 mg/pound (usually 1 ml/10 pounds)
Circulatory compromised: 39 mg/pound
(usually 1 ml/10 pounds)
Needle insertion point: right or left side,
4th intercostal space, or sternally in cats
Syringe retracts: large volume (fill) of blood
Injection speed: slow (0.5–1 ml/second)
and consistent with good technique
Time to cessation of heartbeat (death):
5–10 seconds
Time to cardiac standstill (no fibrillation):
~2–5 minutes
The proper location for intracardiac injection or heart stick

30
The Humane Society of the United States Euthanasia Reference Manual
Oral Administration of
Sodium Pentobarbital (PO)
Where permitted by law, oral administration of
sodium pentobarbital can be particularly useful
for very dicult or fractious animals. For exam-
ple, if a dog is especially aggressive and willing
to charge the front of its kennel, it can be much
easier and safer to simply squirt sodium pento-
barbital directly into his or her mouth or put the
drug into some wet food and allow the animal
to eat it rather than try to put the dog on a
control pole and attempt injection. However,
it can be dicult to get an animal to ingest
sodium pentobarbital because of its bitter taste,
and squirting the drug into a moving target like
a snarling animal’s mouth can be tricky. Most
often, the animal ingests only a small quantity
of drug—it is rare that an animal will ingest
enough to achieve euthanasia. However, the
goal of oral administration of sodium pento-
barbital is usually not to fully complete the
euthanasia process but to simply get the animal
to ingest enough drug to render him fully
unconscious for direct injection of additional
sodium pentobarbital, or at least manageable
enough to be put in a net or on a control pole
for safe injection of a pre-euthanasia drug.
In such cases, injection of additional sodium
pentobarbital should follow once the animal
has lost consciousness, in order to ensure that a
sucient dose to achieve euthanasia is received.
Oral administration of sodium pentobarbital is
limited because:
• It is expressly prohibited in certain states
• The drug has a bitter taste, so many animals
(cats in particular) will not ingest it
• It has a high dosage requirement (3 times
greater than the IV dose)
Sodium Pentobarbital
Oral Administration
(PO, per os, Latin for “by mouth”)
(squirting drug directly into animal’s
mouth or mixing it into food)
Primary Advantages
• Useful for aggressive, fractious animals
that are unsafe to handle.
Primary Disadvantages
• Expressly prohibited in certain states.
• The drug has a bitter taste, making it
dicult to get a sucient amount of
the drug ingested to achieve death
(therefore it will most likely have to be
followed up with IV or IC injection).
• Should be limited to rare cases of
extremely fractious animals that cannot
be safely and humanely handled by any
other means.

31
The Humane Society of the United States Euthanasia Reference Manual
CHAPTER 3
Pre-Euthanasia Drugs
Ideally, every animal scheduled for eutha-
nasia could be gently restrained for direct
IV or IP injection of sodium pentobarbital.
However, in reality not every animal can be
safely handled with gentle physical restraint,
and in some cases animals are so unsocialized
or fearful that attempts at physical handling
would sharply increase their level of stress. For
those animals, pre-euthanasia drugs should
be administered to render them uncon-
scious before the lethal injection of sodium
pentobarbital. Availability of the proper
pre-euthanasia drugs and proper training
regarding their use are therefore integral to
the success of any euthanasia protocol.
Should all animals be anesthetized
before euthanasia? Not necessarily.
The notion that administration of pre-
euthanasia drugs is more humane than
direct injection for every animal in
every circumstance is incorrect. For
many well-socialized animals, direct
injection of sodium pentobarbital is
perfectly humane because it can be
achieved virtually painlessly.
Advantages
The primary advantage of pre-euthanasia drugs
is that their use allows the euthanasia techni-
cian to render the animal unconscious without
requiring the same level of human contact
necessary for direct administration of sodium
pentobarbital. A fractious or fearful animal
may be physically restrained for intramuscular
(IM) injection of a pre-euthanasia drug with a
control pole, squeeze cage, restraint gate, feral
cat box, or other remote device, injected safely,
then released to a quiet, low-stress environ-
ment while the drug takes eect. This release
period allows the animal to relax, with the sense
that the human interaction is over; the next
time the animal is touched by human hands,
he will be unconscious. Alternatively, some
pre-euthanasia drugs can be administered with-
out any contact or handling at all (including
anesthetic inhalants, or oral administration of
certain drugs). Once the animal has lost con-
sciousness, the technician can safely handle the
animal for injection of sodium pentobarbital
without causing undue stress or discomfort.
Disadvantages
Pre-euthanasia drugs do have significant
disadvantages that should prevent shelters
from making their use mandatory for every
animal. Most significant, injection of pre-
euthanasia drugs is painful for animals, even
when done properly. The low pH of the drug
and the volume of the solution forced into
densely packed muscle tissue during an IM
injection causes a stinging sensation for every
animal; when the drug is improperly injected
outside muscle tissue, or when the animal is
so emaciated that there is barely any muscle
tissue available for injection, administration of
pre-euthanasia drugs can cause considerable
pain. Moreover, all pre-euthanasia drugs have
potential side eects, and two animals of the
same breed and size may react very dierently
to the same type and dosage of drug, making
administration somewhat complicated. In
fact, if the wrong drug is selected, or if the
animal has an unanticipated reaction to the
drug, the animal can actually become more
dangerous. This is especially concerning since
Pre-Euthanasia Drugs

32
The Humane Society of the United States Euthanasia Reference Manual
routine use of pre-euthanasia drugs may give
sta a false sense of security when working
around fractious or aggressive animals, or
they may begin to rely on the use of drugs
as a substitute for safe and compassionate
handling of every animal.
Finally, many pre-euthanasia drugs are very
expensive, and some are controlled substances
that must be handled and secured just as strictly
as sodium pentobarbital to ensure compliance
with state and federal laws. Many pre-eutha-
nasia drugs are restricted by state law to use
by or on the order of a licensed veterinarian.
Shelters without a veterinarian on sta should
work closely with a local veterinarian to create
guidelines and procedures for the use of drugs,
and must be careful to honor their relationship
with that veterinarian by strictly complying with
those guidelines and procedures.
Policy
For the reasons noted above, blanket policies
requiring all animals to be given pre-euthana-
sia drugs are not always in the best interest of
the animals or the organization, and should be
carefully reconsidered. When appropriate, it
is often best practice to hold and comfort an
animal for direct IV or IP injection of sodium
pentobarbital rather than injecting a pre-
euthanasia anesthetic, but neglecting or refus-
ing to use pre-euthanasia drugs when direct
injection would cause the animal undue stress
is equally ill-advised. A technician should not
hesitate to use a pre-euthanasia drug when
there is a clear need; no animal should ever
be forced or excessively restrained for direct
injection of sodium pentobarbital, for not only
is that inhumane for the animal, it puts sta
at unreasonable risk of serious injury. A good
euthanasia technician must have a variety of
options at his or her disposal and must be able
to adapt to changing circumstances, and it is
sound policy to allow technicians the discre-
tion to choose the drugs and techniques most
appropriate for each individual animal based
on thorough training and the advice and guid-
ance of consulting veterinarians.
Pre-Euthanasia Drugs
As a rule, all aspects of euthanasia should
be performed out of sight and hearing of
other animals (as well as members of the
public and volunteers); even the admin-
istration of pre-euthanasia anesthetics
is optimally performed in a separate
euthanasia room. However, in some
cases putting an animal in a net or on a
catchpole and forcing him or her into the
euthanasia room for injection of a pre-eu-
thanasia drug may put employee safety
at risk and may cause undue distress, not
just for that animal but for all of the other
animals who see and hear the struggle.
In those cases, discretion may dictate
that the animal be anesthetized inside a
kennel, then transported to the eutha-
nasia room after loss of consciousness.
Care must be taken, however, to limit the
exposure of other animals to any portion
of the euthanasia process to the greatest
extent possible (for example, blankets
and towels can be eective ways to block
animals’ views, etc.).
Vaccines are administered under the
skin (SQ), pre-euthanasia drugs into
muscle (IM) (typically), and sodium
pentobarbital into veins (IV)—but why?
The appropriate routes of administration
for each drug are clearly marked on the
label, and are based on factors like how
quickly the drug needs to be circulated
for maximum eect (for example, vac-
cines should be absorbed more slowly
than other types of drugs, so they are
administered SQ). Administration of
any drug in a manner not prescribed by
the manufacturer’s label is unlawful in
the United States.

33
The Humane Society of the United States Euthanasia Reference Manual
Types of Pre-
Euthanasia Drugs
Just as important as the decision whether or
not to use a pre-euthanasia drug is the selec-
tion of the most appropriate drug. The best
pre-euthanasia drugs are anesthetics, drugs
that quickly render the animal unconscious with
a total loss of ability to feel pain and that have
minimal side eects. Although no drug is per-
fect, for dogs and cats, Telazol or a combination
of the drugs xylazine and ketamine (commonly
referred to as “PreMix”) are currently the pre-
ferred drugs for pre-euthanasia (there may
be newer drugs that can be considered accept-
able for pre-euthanasia in the near future;
their use must be evaluated by a veterinarian
to ensure that they are as humane and eective
as Telazol and PreMix).
Best Pre-Euthanasia Drug
Option A: PreMix
(Xylazine/Ketamine Combination)
When the drugs xylazine and ketamine are
used in combination at appropriate dosages
(this is commonly referred to in the United
States as “PreMix”), the animal is put into a
deep plane of anesthesia—unconscious, unable
to move, and unresponsive to even deep pain
stimuli. The animal has no blink reflex or toe-
pinch reflex, but still retains good circulation
and blood pressure (meaning sodium pento-
barbital can be administered intravenously
safely and eectively).
While PreMix is in most cases a very good
choice for pre-euthanasia anesthesia, euthana-
sia technicians must be aware of its potential
drawbacks. The animal does experience a
stinging sensation when any drug is adminis-
tered intramuscularly, so care must be taken
to inject properly. Injecting slowly into a large
muscle mass (the back muscle is ideal, since it
ensures smooth absorption of the drug; also
acceptable is the large muscle of the back leg)
is the best way to alleviate pain to the extent
possible. PreMix does have the potential to
induce seizures or vomiting, so the animal must
be closely monitored after administration.
Finally, there are administrative complications
with using PreMix– ketamine is federally
controlled, so the resulting combination of
the two drugs must be treated as a controlled
substance (see Chapter 10). Moreover, in
some states the mixing of two such drugs is
considered to be “compounding,” and as such
may violate applicable state board of phar-
macy regulations.
To make PreMix, first ensure that xylazine
and ketamine are added together at a ratio
of one part xylazine to five parts ketamine
(for example, add 2 milliliters of large-animal
xylazine (1 milligram per pound, 100 milligrams
per milliliter) to a standard 10-milliliter vial of
ketamine (5 milligrams per pound), creating a
12-milliliter bottle of PreMix that can be dosed
for individual animals as needed). IMPORTANT
NOTE: Ensure that a new label is placed onto
the vial of mixed drugs that specifies the
names of the drugs mixed, the amounts of
each drug, the date mixed, and the initials
of the individual who prepared the mixture.
Pre-Euthanasia Drugs
PreMix bottle, labeled

34
The Humane Society of the United States Euthanasia Reference Manual
The proper dosage for administration of most
formulations of PreMix as a pre-euthanasia
anesthetic is 0.5 milliliter for every 10 pounds
the animal weighs, given intramuscularly. An
animal that has been administered PreMix
will generally feel the full eects of the drugs
within three to five minutes, and will remain
anesthetized for 30 to 40 minutes before
regaining consciousness.
Pre-euthanasia drugs should not be
“dosed for eect”—the specified amount
should be given by weight regardless of
whether the animal is pregnant, elderly,
heavily muscled, etc.
Remember, because ketamine is a Schedule
III controlled substance, be sure to record and
track the use of this combination product in
detail in the controlled substance logbook,
as required by law.
Best Pre-Euthanasia
Drug Option B: Telazol
(Tiletamine/Zolazepam Combination)
Like PreMix, Telazol also provides deep anes-
thesia in which the animal quickly becomes
unconscious, immobile and unable to feel pain,
allowing for all routes of injection of sodium
pentobarbital. It is particularly recommended
as a pre-euthanasia drug because it stings less
than PreMix does on injection and is less likely
to induce seizures.
Telazol is the trade name for a drug combi-
nation of tiletamine and zolazepam, and is
used as an anesthetic in veterinary practice for
minor surgical procedures because it provides
rapid unconsciousness, analgesia and good
muscle relaxation. The drug begins to take
eect within 10 minutes of IM administration
and lasts for approximately 30 minutes before
the animal starts to wake up. These properties
also make Telazol a good choice for chemical
capture of animals in the field.
Although Telazol is an excellent choice as a
pre-euthanasia drug it can be fairly expensive,
and it has a short shelf life once it has been
reconstituted (meaning sterile water has
been added); it must also be refrigerated to
maintain potency for more than a few days.
These are two important considerations that a
shelter should weigh before selecting Telazol
as the only pre-euthanasia drug kept available
for dogs and cats; many shelters elect to use
PreMix for dogs and reserve Telazol for cats,
because the lower doses required for cats
alone make it much more cost-eective.
Telazol typically comes in powder form in small
5-milliliter bottles and must be reconstituted
with 5 milliliters of sterile water. Draw 5 milli-
liters of sterile water into a syringe and inject
it directly into the bottle of powdered Telazol,
then roll or gently shake the bottle to ensure
thorough mixing. It is important to label each
bottle of Telazol with the date it was recon-
stituted to ensure that its expiration date is
apparent. The reconstituted solution remains
eective for only four days when stored at room
Pre-Euthanasia Drugs
Telazol

35
The Humane Society of the United States Euthanasia Reference Manual
temperature; it can last up to 14 days when
refrigerated. IMPORTANT NOTE: Refrigeration
may not be an option without a means to
securely store the drug (see Chapter 10).
In most settings, Telazol, like PreMix, should be
administered by IM injection into the back mus-
cle (epaxial) or rear thigh muscle (bicep femoris)
of the animal (in certain cases subcutaneous
administration can be performed, but for use in
companion animals IM injection is preferred).
While the injection does sting, it is not quite as
painful as injection of other drugs. The label
dose of Telazol is 0.3 to 0.45 milliliters for every
10 pounds of animal, given intramuscularly;
however, best practice is to use .5 milliliters per
10 pounds to achieve full analgesic eect.
Telazol is a Schedule III controlled substance
and must be strictly handled and secured to
comply with federal, state, and local laws.
Other Pre-Euthanasia Drugs:
Conditionally Acceptable,
but Not Preferred
A variety of other drugs can be used for pre-
euthanasia when direct injection of sodium
pentobarbital is not feasible, but because of
their side eects or their lack of analgesic prop-
erties (they fail to provide sucient pain relief
or full unconsciousness), or both, they are not
as preferred as either Telazol or PreMix.
Acepromazine/ACE (PromAce)
Acepromazine (“ACE”) is a mild tranquilizing
agent that depresses the central nervous
system. It is typically used to prevent vomiting
during surgery, in conjunction with other
drugs. An animal experiencing the eects of
ACE may have some diculty maintaining
balance, but will usually remain on his or her
feet, appearing conscious but subdued, moving
in slow motion. The animal remains somewhat
aware of what is taking place around him or
her, and may react suddenly to loud noises or
quick movements.
Because ACE is a tranquilizer, rather than an
anesthetic, it is not recommended for use as
a pre-euthanasia drug by itself. Not only can
it induce seizures, in some cases it can make
the animal more excited, unpredictable, and
dangerous because it may act as a stimulant
rather than a tranquilizer, and may reduce an
animal’s natural bite inhibition (so an animal
Pre-Euthanasia Drugs
Dosage Chart for
Telazol
®
and PreMix
Animal’s Weight
(pounds)
Milliliters
(ml)
5 0.25
10 0.5
15 0.75
20 1
25 1.25
30 1.5
35 1.75
40 2
45 2.25
50 2.5
55 2.75
60 3
65 3.25
70 3.5
75 3.75
80 4
85 4.25
90 4.5
95 4.75
100 (and up) 5
Increasing the dosage of a pre-euthanasia
drug generally does not speed up the
drug’s eects, it only extends the length
of time that the animal feels the influ-
ence of the drug.

36
The Humane Society of the United States Euthanasia Reference Manual
who typically would not bite may do so while
under the influence of the drug). An animal
that is highly stimulated but unable to inhibit its
behavior can prove very dangerous indeed, and
is capable of causing serious injury. Therefore,
ACE should never be used alone; it should be
used only in conjunction with a drug with true
anesthetic properties. Moreover, ACE has been
shown to have negative eects when given
before injections of sodium pentobarbital
combined with the drug phenytoin (in the drug
Euthasol, for example) because it diminishes the
drug’s eectiveness at slowing the heart.
ACE can be administered via subcutaneous (SQ)
or IM injection, and takes 10 to 20 minutes for
full eect. ACE is also available in tablet form
(in doses of 5, 10, or 25 milligrams), so that
the drug can be mixed in a treat ball or with
canned food for oral administration. When
administered orally it can take 30 to 40 minutes
for full eect.
The general dosages for ACE (10 milligrams/
milliliters, small animal product) are:
Subcutaneous or IM injection: 0.1 milliliter
per 10 pounds of body weight (3 milligrams
maximum total dose for dogs, 1 milligram
maximum for cats); oral administration of ACE
pills: 1 milligram per pound of body weight.
Xylazine
Xylazine (commercially available under the
names Rompun and AnaSed, among others) is
a non-narcotic sedative, analgesic, and muscle
relaxant; it is not a true anesthetic because
it does not render the animal unconscious. It
does, however, act as a central nervous system
depressant, rendering most dogs and cats
somewhat immobile and producing a moder-
ate level of short-term analgesia (pain relief),
typically for about 30 minutes. The result is
an animal that is heavily sedated and thus
easily controllable for giving an IV injection
(although good restraint technique should
always continue to be practiced even when an
animal appears heavily sedated). Xylazine is
available in two concentrations: large animal
(100 milligrams per milliliter) and small animal
(20 milligrams per milliliter). Most shelters use
the large animal formulation for dogs and
cats because it is more cost-eective. Another
benefit of xylazine is that, unlike other pre-
euthanasia drugs, it is not a controlled
substance subject to stringent federal and
state storage and tracking requirements.
Nevertheless, it is good standard practice to
record the purchase and use of xylazine as
with any controlled drug.
Despite these advantages, xylazine is not
recommended for use as a pre-euthanasia
drug by itself because: a) it commonly causes
vomiting, particularly in cats and in any animal
that has recently eaten; b) though sedated,
the animal remains conscious, and may react
violently to sudden noises and movements; c)
it may dangerously reduce the animal’s natu-
ral bite inhibition, making it potentially even
more dangerous to handle; and d) it lowers
the animal’s blood pressure to the point that
it can be dicult to inject the sodium pen-
tobarbital for euthanasia. For these reasons,
xylazine is recommended for use only when
combined with another drug (like ketamine
to create PreMix, above), that tempers these
negative eects.
Using xylazine alone is particularly
dangerous when working with horses,
because it tends to make them more
likely to kick.
Ketamine
Ketamine (available commercially as Ketaset,
Ketaject, and others) is an anesthetic agent
that renders an animal completely immobile.
However, when used alone it can cause the
muscles to become rigid, causing the body to
Pre-Euthanasia Drugs

37
The Humane Society of the United States Euthanasia Reference Manual
stien. It also stings so much upon injection
that it creates a fairly pronounced reaction in
most animals. Moreover, in large doses it can
produce convulsions and seizures. For these
reasons, ketamine is recommended for use
only when combined with another drug (like
xylazine to create PreMix, above), that tempers
these negative eects.
Shelters using ketamine either alone or in
combination should also be aware that it has
a strong potential for abuse by humans because
of its profound dissociative eects. Ketamine
is a Schedule III controlled substance and must
be handled and secured strictly according to
federal, state, and local laws, similar to sodium
pentobarbital. Shelters are advised to take
additional measures as needed to prevent
against theft and abuse.
Administration of
Pre-Euthanasia Drugs
Most pre-euthanasia drugs are injected directly
into the animal’s muscle tissue (intramuscular, or
IM, injection), typically using a small-gauge nee-
dle to help reduce the sting and ensure that the
drug is injected into muscle, not bone. The most
common location for this type of injection is in
the lumbar muscle along the spine (epaxial) or
the large muscle of the rear thigh (biceps femo-
ris). Each of the pre-euthanasia drugs discussed
above produces a distinct stinging sensation on
administration, which is likely to cause even the
most cooperative animal to react. Just as is the
case with direct injection of sodium pentobar-
bital, each animal should be gently but securely
restrained before an IM injection. However,
unlike direct injection of sodium pentobarbital
where a bolus eect is preferred (injection of
a large quantity of drug at one time), when
injecting pre-euthanasia drugs it is important
to inject slowly in order to minimize the pain
caused by the drug entering the muscle tissue;
an experienced euthanasia technician will look
to balance the need to inject slowly, to minimize
the animal’s pain, with the desire to finish the
process quickly so as to lessen the stress induced
by handling the animal. IMPORTANT NOTE:
Pre-euthanasia drugs are typically administered
to animals that are fractious or feral, making
accurate placement of an injection dicult.
Nevertheless, it is unacceptable to simply
inject an animal randomly in any accessible
location—euthanasia technicians must ensure
that IM injections are made only into muscle
tissue. Tools like restraint gates should be used
to ensure that the animal is suciently immobi-
lized to allow for accurate injection (see Chapter
8 on humane animal handling).
Standard syringes (for example, 1cc or 3cc)
and small-gauge needles are typically used to
administer pre-euthanasia drugs, depending
on the volume needed. The injection should
Pre-Euthanasia Drugs
Muscle masses in rear leg of dog, appropriate
for IM injection of pre-euthanasia drugs
Gluteus
medius
Epaxial muscles
Gluteus
superficialis
Semilendinosus
Sartorius
Tensor fasciae
latae
Semimembranosus
Biceps
femoris

38
The Humane Society of the United States Euthanasia Reference Manual
be delivered at a 90-degree angle to the skin,
directly into the largest accessible muscle mass
(typically the thigh of the rear leg). The tech-
nician holds the syringe with one hand, with
thumb ready to push the plunger and gently
but firmly grasps the leg to be injected with the
other hand to help steady the animal. He then
inserts the needle and injects the full amount
of the drug as slowly as the animal will permit.
In some cases it may be excessively dicult or
dangerous to use a traditional syringe. Each
shelter should have at least one alternative
injection device, such as a Safety Stick™, if avail-
able. A Safety Stick is a fairly inexpensive piece
of equipment that may be used to safely and
humanely administer a pre-euthanasia injec-
tion to a wild or fractious animal. The syringe,
at the end of a 3- to 5-foot pole, is filled with
the drug just as it would be for a normal injec-
tion. The stick simply serves as an extension of
the technician’s arm, allowing him to adminis-
ter the drugs from the plunger on the end of
the stick while maintaining a safe distance from
the animal. A short but stout needle is inserted
into the muscle of the animal’s hindquarters,
and the plunger on the end of the Safety Stick
is pushed gently to inject the drug. The ani-
mal is immediately released afterward into a
secure, dark, quiet area while the drug begins
to take eect.
Some agencies still use traditional pole syringes,
in which there is no plunger to be depressed by
the technician, rather the jabbing motion itself
is used to push the drug through the syringe
(akin to a harpoon). A technician operating a
traditional pole syringe has much less control
over the injection process than that available
when using a Safety Stick, and is more likely to
induce pain to the animal, so Safety Sticks are
preferable. If only a traditional pole syringe is
available, a technician can enhance the level of
control over injection by looping the ring of a
traditional flat leash over the needle and using
a slow but steady pulling motion to regulate
the rate of drug injection (as an alternative, a
rubber stopper, like those used to block the end
of a test tube, can be applied over the needle
to provide a base of resistance and limit how
much needle can pierce the animal’s skin, but
the very act of applying the stopper will dull the
needle). IMPORTANT NOTE: As stated above, an
IM injection must be delivered into muscle—it is
not permissible to simply inject into any accessi-
ble skin of the animal. Trying to accurately inject
an animal with a Safety Stick pole syringe while
he or she is loose in a cage is nearly impossi-
ble; therefore, animals that will be injected
via Safety Stick or pole syringe should first be
confined to a smaller space using a press gate,
squeeze cage, or other device so that injection
can be performed accurately. It is never appro-
priate to simply stab at an unsecured animal
and possibly inject into a non-muscled area.
Pre-Euthanasia Drugs
Use of a Plexiglass
®
Shield to secure a cat for injection
When using a Safety Stick™ or a pole
syringe, it can be dicult to judge how
much pressure is required to firmly insert
the needle without causing undue pain.
Practice injecting on a ripe banana—the
goal should be to cleanly pierce the peel
without bruising or squishing the banana.

39
The Humane Society of the United States Euthanasia Reference Manual
As the animal begins to feel the eects of
the drug, he or she will typically start to lick
the lips, as though experiencing an unpleas-
ant taste in the mouth, lose the ability to
focus vision (the eyes will often dart from
one side to the other, as though the animal
is watching a tennis match), and then lose
coordination and begin to drop to the floor.
Although the animal is not experiencing any
pain during this time, the handler should
take care to ensure that he does not stumble
into equipment or otherwise injure him or
herself and that he drops gently to the floor.
The technician should also monitor for any
seizures or vomiting (in which case it is critical
to ensure that the animal does not choke on
its own vomit before sodium pentobarbital
can be humanely administered). IMPORTANT
NOTE: Just as with sodium pentobarbital,
while the animal is experiencing the eects
of a pre-euthanasia drug he or she can enter
an excitement phase and lose normal bite
inhibition—handlers must ensure that they
and other sta members remain safe until the
animal has fully lost consciousness.
Inhalant Anesthetics
(Halothane, Isoflurane)
When euthanizing very small rodents or birds,
injection can be extremely challenging. In
such cases, liquid inhalant anesthetics like
halothane or isoflurane can be used to induce
rapid unconsciousness and death. Inhalants
work on the principle of displacing oxygen with
an alternate gas; because the body’s organs
require oxygen to function, when another gas
is inhaled, the body begins to shut down, and
death is inevitable. Whether or not this death
is considered to be humane depends on what
happens between the time that the animal
first inhales the gas and the time consciousness
is lost. If the process is almost immediate and
completely pain-free, death can be considered
humane; however, if the animal experiences
any physical or psychological distress or suf-
fers in any way before losing consciousness,
the death is inhumane. Many variables aect
whether a death by inhalant is humane, includ-
ing the physical eects of the inhalant itself,
the method of delivery, and even the animal’s
reaction to the inhalant.
According to the AVMA, halothane is consid-
ered the most eective and humane inhalant
anesthetic. Other inhalant anesthetics like
isoflurane are less desirable because their
pungent odor may cause the animal to ini-
tially hold his or her breath, delaying the
onset of unconsciousness. However, in the
shelter setting, halothane can be dicult to
acquire, so isoflurane is most commonly used.
Chloroform and ether are not acceptable
inhalants for use in euthanasia in part because
of the risk of bodily harm they pose to humans
(they are a possible carcinogen and can cause
liver damage).
Pre-Euthanasia Drugs
Injecting fractious animals can be intimi-
dating even for experienced technicians.
Bending or stooping to inject an animal in
the muscle of the leg places the technician
in a very vulnerable position even under
the best of circumstances, but grabbing
the leg of a large, snarling dog held in
place only by a restraint gate or the slim
cable of a control pole takes a great deal
of courage. In such cases, trust between
the handler and technician is paramount.
The technician must trust that the han-
dler has a secure hold on the animal and
will not permit him or her to be bitten;
likewise, the handler must trust that
the technician will inject quickly, calmly,
and eectively, and will not escalate the
situation by panicking. This trust is only
built up over time, and euthanasia teams
should have the freedom to discuss their
fears and concerns, practice handling ani-
mals together, and build a solid working
relationship before they are called on to
tackle dangerous animals.

40
The Humane Society of the United States Euthanasia Reference Manual
Technically speaking, inhalant anesthetics
can be used either as direct euthanasia
agents or as pre-euthanasia drugs. However,
it is advisable that an appropriate dose of
sodium pentobarbital be injected into animals
that have been rendered unconscious with
the use of an inhalant anesthetic in order
to ensure that death is achieved as rapidly
as possible.
IMPORTANT NOTE: Liquid inhalant anesthet-
ics must never be allowed to directly contact
animals because they can cause irritation;
rather, the liquid should be poured onto cotton
balls, gauze, or other absorbent materials and
placed with the animal in an airtight container,
so that the animal is exposed only to the vapors
produced, rather than the liquid itself. Inhalant
agents are generally not federally regulated
controlled substances; nevertheless, it is good
practice to control their storage and use in
the same manner as other euthanasia drugs.
Leftover absorbent materials must be safely
disposed of so that there is no unnecessary
human exposure to the inhalant.
Pre-Euthanasia Drugs

41
The Humane Society of the United States Euthanasia Reference Manual
CHAPTER 4
Verification of Death—
The Most Critical Step in
the Euthanasia Process
The euthanasia process is not complete until
death has been verified. Dramatic stories
about euthanized animals “waking up” after
euthanasia are not modern-day miracles, but
examples of grievous failures in the euthanasia
process—the animal was not administered an
amount of drug sucient to depress essential
brain functions, and so never moved from Stage
III, surgical anesthesia, into Stage IV, medul-
lary paralysis; in eect, the animal was never
actually euthanized at all. If the technician had
properly tried to verify death he would have
discovered that the animal’s basic life functions
had not been completely suppressed, and
would have recognized that administration of
additional sodium pentobarbital was necessary
to achieve euthanasia.
Unless proper verification steps are taken, it
is surprisingly easy to mistake an animal for
dead. Sodium pentobarbital is a potent drug
that puts animals into a deep anesthetic plane
that can fool even longtime technicians. The
animal can appear lifeless, without any evi-
dence of breathing or heartbeat, yet still retain
minimal life-sustaining functions. The animal
may survive in this state until the drug wears
o, sometimes many hours later, and then will
appear to “revive.” Because episodes like this
are possible, it is essential to develop a protocol
for the verification of death and ensure that it is
followed for every animal, without exception.
It is not an exaggeration to say that the implica-
tions of failing to verify death are monumental.
The trauma for the animal who “wakes up” in
a landfill, freezer, or worse, is unimaginable,
and the guilt experienced by the euthanasia
technician responsible can be overwhelming.
Moreover, media reporting of such an incident
can do lasting damage to the reputation of a
shelter, and the loss of the public’s trust can be
permanent. For all these reasons, verification of
death can be viewed as the most crucial aspect
of the euthanasia process, and the eutha-
nasia technician must take responsibility for
checking each animal and ensuring that death
has in fact occurred. If there is any doubt, the
animal should be injected again with sodium
pentobarbital and rechecked until death is
definitively confirmed. Only at that point
should proper disposal of the animal’s body be
made, in compliance with policy and with state
and local laws.
Steps for Verifying Death
First: Ensure that the animal has neither a
blink reflex nor a toe pinch reflex.
Second: Use a stethoscope to verify that
respiration has stopped.
Third: Perform a cardiac stick or verify the
onset of rigor mortis.
Verification of Death

42
The Humane Society of the United States Euthanasia Reference Manual
Verifying Death
When an animal “wakes up” from euthanasia it
is typically because the technician has misinter-
preted two signs, the appearance of lifelessness
and lack of breathing, as proof of death. It is
critical for euthanasia technicians to understand
why these two signs can be deceptive, and why
armative steps must be taken to definitively
declare an animal dead.
As we know from the discussion of the stages
of anesthesia above, sodium pentobarbital
puts animals into such a deep plane of surgical
anesthesia that they appear to the naked eye to
be completely lifeless. They are so completely
unresponsive to any stimuli that they have
lost both their palpebral (blink) and toe-pinch
reflexes. Nevertheless, they may still be only
in Stage III, surgical anesthesia, and their vital
functions have not been completely depressed.
Therefore, an appearance of lifelessness and
lack of reflexes alone are not sucient to con-
clude that an animal has died.
Similarly, the apparent lack of breathing alone
cannot be definitively relied upon. While an
animal cannot survive without breathing
oxygen, an animal in Stage III anesthesia may
take breaths that are so shallow and infrequent
that they can be missed by mere observation. A
stethoscope must be used to ensure that there
is in fact no heartbeat. It is important to note,
however, that untrained ears, particularly when
using an inexpensive stethoscope, may miss
a very faint, weak heartbeat—even on a fully
conscious, 100-pound, overweight dog that
is standing up with his tail wagging! Imagine,
then, how dicult it can be to determine the
absence of heartbeat in a surgically unconscious
animal. For this reason, euthanasia techni-
cians must receive proper training in using
a stethoscope, and must have high-quality,
reliable equipment.
To be 100 percent accurate in determining
death, technicians should consider supplement-
ing the use of a stethoscope with one of two
additional methods: a) waiting several hours
for rigor mortis to set in; or b) using a method
called cardiac stick (or heart stick) to determine
that the animal’s heart has ceased beating.
While either method is acceptable, the presence
of rigor mortis is the only indisputable proof
of death; however, in the shelter environment,
where time is often of the essence, cardiac stick
is most commonly used, and when performed
correctly is equally as accurate. IMPORTANT
NOTE: In some states an animal may not be
left unattended until death has been verified;
ensure that you are familiar with the laws appli-
cable in your state before you leave the animal
while awaiting rigor mortis.
A stethoscope is one means of verifying death
If the animal does not appear to be
breathing regularly but instead appears
to be occasionally gasping, the animal is
exhibiting what is referred to as “agonal
breathing.” These are not actual attempts
by the animal to breathe, because the
animal is not actually gasping in an eort
to bring air into his or her lungs; rather it
is a reflex reaction to the lack of oxygen,
and is a normal part of the dying process.
Verification of Death

43
The Humane Society of the United States Euthanasia Reference Manual
Performing a Cardiac
Stick (‘Heart Stick’)
A cardiac stick involves the insertion of a needle
and syringe directly into the heart of the fully
unconscious animal (IMPORTANT NOTE: A
needle must never be inserted into the heart of
a conscious animal—the euthanasia technician
must be certain that the animal has no blink
or toe-pinch reflexes before the cardiac stick is
performed). Once the needle has pierced the
heart muscle, any movement of that muscle
will be transferred to the syringe, which will
then mimic the heartbeat extremely accurately.
Imagine that the animal’s heart is the base
weight of a metronome, and the syringe is
the metronome’s pendulum—when the heart
muscle moves the base of the metronome, the
syringe protruding from the base will swing
in response. If the syringe reflects zero move-
ment (assuming it was properly placed in the
heart muscle), it can be definitively determined
that the heart has ceased beating, and death
can be confirmed.
It is important to note that every movement
of the syringe does not necessarily mean the
animal is actually alive; an experienced eutha-
nasia technician can tell the dierence between
movement that indicates a true heartbeat,
meaning the heart is still circulating blood
through the body, and mere fibrillation. If
the syringe is moving in a circular fashion, the
heart is beating and circulating blood, and
the animal is still technically alive; in this case,
either the technician should wait a few minutes
longer to allow the drug additional time to
take eect or administer additional sodium
pentobarbital to the animal to facilitate death.
If, on the other hand, the needle is moving in
a side to side fashion (like a true metronome),
the heart muscle is simply fibrillating, twitching
or spasming aimlessly, and is not circulating
blood. In this case, the animal is technically
dead; nevertheless, the euthanasia technician
should wait until the fibrillation has ceased and
the syringe is still before declaring the animal
dead. (IMPORTANT NOTE: Injecting more drug
into a heart that is fibrillating does not have
any eect, as the heart is no longer capable of
pumping that drug to the brain—the technician
should just wait as long as necessary for the
heart to become still).
To perform a cardiac stick, one uses essentially
the same technique as for giving an IC injec-
tion (see Chapter 2), except that the syringe
attached to the needle is empty. Unlike IC injec-
tion, however, the needle need not be inserted
into a chamber of the heart; any portion of the
heart muscle will do. As long as some blood is
drawn into the syringe upon aspiration, either a
small flash or a large surge, the needle is prop-
erly placed in the heart for purposes of a cardiac
stick. If the animal is particularly obese or has a
broad, barrel chest, switching to a longer nee-
dle may be required. Conversely, if the animal is
tiny, the technician will want to use the smallest
needle available; in fact, it may be necessary to
remove the syringe from the needle once the
needle has been placed in the heart in order
to accurately determine whether the heart is
moving, since the syringe may weigh the needle
down and prevent the technician from seeing
movement. Be aware that once the syringe is
removed there may be a discharge of blood
through the open hub of the needle.
Even though a cardiac stick is the best method
of verifying death aside from rigor mortis, it
is not generally used when the euthanasia
is being viewed by the animal’s owner or by
another member of the public. Although the
stick is not conducted until after the euthana-
sia technician has confirmed that the animal
is fully unconscious and completely unable to
feel pain, to the uninitiated, viewing a needle
in the heart may be disturbing. In these situa-
tions, extra care must be taken to ensure that
the determination of death is 100 percent
accurate. In addition to using a stethoscope to
verify the absence of a heartbeat, the techni-
cian should verify that the gums and mucous
membranes of the animal’s mouth have turned
Verification of Death

44
The Humane Society of the United States Euthanasia Reference Manual
bluish or purplish (this is called cyanosis, which
indicates the lack of oxygen in those tissues).
The technician may also test for a lack of rectal
sphincter tone.
If cardiac stick cannot be performed, or as an
additional measure of certainty, confirming
the onset of rigor mortis can determine death.
Rigor mortis is defined as the state of muscle
rigidity that occurs in mammals after death—in
full rigor, the animal will be rendered com-
pletely sti, essentially frozen into the position
in which it died. Signs of rigor mortis can usually
be observed within a half-hour of the injection,
but it could take as many as several hours after
death, depending on the air temperature and
other factors. During this period, the body
should be covered with towels, sheets, or tarps
whenever possible; showing respect for an
animal throughout the complete euthanasia
process is essential. IMPORTANT NOTE: After
about 12 hours the animal will pass out of rigor
mortis and lose the telltale full-body stiness
as the body begins to decompose. Therefore,
it is important to ensure the proper window
of time is factored when using rigor mortis
to verify death.
Verification of Death

45
The Humane Society of the United States Euthanasia Reference Manual
CHAPTER 5
Disposal of Animal Bodies
Several factors determine the best method
of disposal for animal bodies, including the
number of animals handled, state and local ordi-
nances, financial considerations, and local public
acceptance of the various methods available.
Placing animal bodies in a landfill is usually the
most economical method of disposal, but it is
not practical in communities that restrict the
use of their landfill facilities. The public is not
always receptive to disposal of dogs and cats
this way—people do not want to see or smell
animal carcasses decomposing in a dumpster
outside the shelter while they await pickup, nor
do they want to see animal carcasses strewn
about within the landfill. Moreover, there are
confirmed cases of wildlife and stray dogs and
cats dying after ingesting sodium pentobarbi-
tal-filled carcasses that have been scavenged
from a landfill. Therefore, if a landfill is to be
used, the bodies should be bagged or boxed
before disposal (which will help minimize the
chances of scavenging birds and other animals
eating carcasses contaminated with sodium
pentobarbital), and they should be frozen or at
least cooled until such time as they are removed
for transport to the landfill.
Boxing animals before landfill disposal is
much preferred to simply tossing bodies
into a dumpster or landfill, and it need
not be an additional expense—check with
your local supermarket about donations
of used banana or other grocery boxes.
One alternative to the landfill is to engage the
services of a rendering company, which pro-
cesses animal carcasses into fertilizers and other
products. Rendering companies typically charge
considerable fees for pickup service (although
costs vary), but they save sta the time and
unpleasant task of transporting bodies to a
landfill. Shelters must be sensitive, however, to
the fact that the public may not look kindly on
euthanized companion animals being rendered.
Therefore, shelters typically reserve the services
of rendering companies for disposal of large
animals like horses or livestock.
Composting has been proposed as a new dis-
posal option, particularly for disposal of large
animals. However, composting requires careful
layering of substrates and regular mechanical
aeration, and research is still being conducted
into whether composting breaks down drugs
like sodium pentobarbital suciently to be
considered safe.
Perhaps the best option for disposal of animal
bodies is cremation, as it is typically less expen-
sive than rendering (although prices can vary)
and more palatable for the public than use of a
landfill. Some shelters have a crematorium on
site and even oer those services to the public
for their own pets, helping to oset the cost
of maintenance. While convenient, a private
crematorium can be prohibitively expensive (the
equipment itself can cost tens of thousands of
dollars, it uses high volumes of fuel, and it can
have prohibitively high maintenance costs) and it
can require air pollution discharge permits from
local, state, and federal regulatory authorities. As
an alternative, many shelters contract with a pri-
vate crematory company to perform this service
on their behalf. Shelters are generally charged
by the pound for cremation of animal bodies,
and must keep bodies frozen until pickup. Most
important, organizations engaging private
Disposal of Animal Bodies

46
The Humane Society of the United States Euthanasia Reference Manual
crematory services must verify that the company
is reputable; stories of animal bodies being
dumped en masse in remote areas by companies
paid to cremate them do appear, and can hurt
a shelter’s reputation. A reputable crematory
company should provide videotapes or other
documentation as evidence that animals sent for
cremation are properly cremated, and should
welcome on-site visits by shelter representatives.
No matter the disposal method, the animal
must be handled humanely and respectfully
throughout the euthanasia process, from
initial selection as a euthanasia candidate,
through injection and verification of death,
and ultimately through disposal of the body.
It is unacceptable for any person to treat an
animal’s body after death with any less care
and reverence as would have been given while
the animal was alive. A euthanasia technician
who disrespects the body of an animal is likely
suering from burnout, or compassion fatigue.
Retraining and counseling interventions
should be undertaken, and serious consider-
ation should be given to whether that person
should be permitted to continue working with
animals at all.
Disposal of Animal Bodies
Animals suspected of having rabies should be handled and managed by trained personnel,
in accordance with applicable state laws. Even though there has never been a case of human
rabies acquired through the process of collecting and testing specimens, contact with saliva,
mucous membranes, salivary glands, spinal cord tissue and/or brain tissue could be hazardous.
Technicians handling rabies suspect animals must always wear personal protective equipment
(gloves, face/eye shield, gown, etc.) and should have received pre-exposure rabies vaccinations.
General guidelines for preparing specimens for rabies testing are:
• Rabies-suspect animals should be humanely euthanized in a manner that preserves
the brain.
• For small mammals like bats or rodents, the whole body can typically be submitted
to the testing facility. In the case of larger animals like cats and dogs, the head of the
animal should severed at the base of the skull (between the foramen magnum and
the atlas) and submitted for testing, so as not to damage the sections of the brain
required for testing. Livestock, equines, etc. must be handled by a veterinarian
or other individual specially trained to remove the brain stem.
• Axes, hatchets, power saws, etc., should not be used for decapitation, since they
not only create a danger of flying bone and tissue, they also create the potential
for aerosolizing virus-contaminated fluids. Shears and hacksaws are preferred tools.
• Specimens should be kept refrigerated. Avoid freezing rabies-suspect animals, since
the thawing process can delay testing.
• Carefully sanitize or dispose of not only the remainder of the animal’s body but any
surfaces (including soil) that may have been contaminated with bodily fluids.

47
The Humane Society of the United States Euthanasia Reference Manual
CHAPTER 6
Euthanasia Tools
The Euthanasia Area
The ideal euthanasia area is a clean, well-
lighted, well-ventilated, and readily accessible
room away from high-trac areas and used
exclusively for performing euthanasia. Since ani-
mals are sensitive to sights, sounds, and smells,
the area should be constructed and maintained
to minimize these stimuli, which otherwise
may cause unnecessary stress. Euthanasia
areas and animal storage areas should also
ideally be located in areas not accessible to
the public or volunteers, both to preserve
solemnity of the practice for the technicians
and handlers involved and to avoid having the
public experience sights and smells that may be
misinterpreted. Basic handling equipment, first
aid supplies, and a variety of needles, syringes,
and other tools should be readily accessible,
as should enclosures designed to safely house
animals in the process of losing consciousness.
An appropriate environment, along with
competent, gentle handling techniques, can
go a long way toward keeping animals relaxed
and calm. As noted elsewhere in this manual,
a relaxed animal can frequently be euthanized
without pre-euthanasia drugs, a benefit for
everyone involved.
Layout and Design
It is highly recommended that each shelter
reserve a room specifically for conducting
euthanasia. It is important to isolate the area
from other shelter activities as much as possi-
ble to reduce the stress on the animals and to
provide the sta with a safe and quiet working
area. A well-appointed euthanasia room should
have adequate space for at least two people
and an animal to move around freely. Exam
tables, cabinets, and other fixtures should be
thoughtfully located to make for an ecient
and comfortable workspace.
In smaller shelters, it may not be possible to
devote a room exclusively to euthanasia. In such
cases, the euthanasia area should be a section
of the shelter that can be separated from the
rest of the building by a partition or curtain.
All other types of activities must be curtailed in
that space while euthanasia is being performed.
Since animals with all stages of contagious
diseases will enter the euthanasia area, it is
crucial that no healthy animals pass through
it on their way to or from other areas of the
shelter. A separate door to the outside is ideal
so that injured, sick, or quarantined animals
can be brought directly into the room without
having to pass through live animal holding
areas. Ideally, the separate door should also
provide access to a cooler, freezer, or vehicle
for the removal of bodies.
Euthanasia Tools
A typical euthanasia room

48
The Humane Society of the United States Euthanasia Reference Manual
A euthanasia room does not have to be a
drab and somber place. It is very important
to create an environment that is as comfort-
able as possible for the people who work
there. The use of skylights, windows (provided
they that do not open onto public areas),
and inviting colors on the walls may help
foster a more soothing mood. Some shelters
have murals, plants, posters, and quiet music
in their euthanasia rooms. If there is a tele-
phone in the room, the ringer should be
turned o (as should personal cellphones),
since ringing and beeping can startle an
animal during the euthanasia process. These
considerations can help reduce some of the
stress on the sta, which translates into less
stress for the animals. They can also provide
a more appropriate atmosphere for a pet
owner who wants to be with his or her
animal during the procedure, if the organi-
zation permits it.
Lighting and General
Environment
Lighting that is bright enough to provide
clear visibility in all areas of the euthanasia
room is very important, since shadows or
dark areas may obscure the technician’s
view of a vein and make it dicult for the
handler to observe the animal’s behavior.
Fluorescent lighting usually provides a
uniform illumination that is not too bright
for most animals’ eyes. Two banks of lights,
one on either side of the work area where
animals are euthanized, provide excellent
illumination with minimal shadows.
The room’s temperature should be within a
reasonable comfort range for humans and
animals. Portable heaters and air conditioners
are sometimes necessary if the room does
not have sucient environmental controls.
Performance is enhanced when animals and
people are comfortable.
It is essential to have good ventilation in the
area to prevent the accumulation of odors.
To minimize the transfer of airborne diseases
into other areas of the shelter, the ventilation
system for the euthanasia room should not be
connected to the rest of the building’s heat-
ing and air conditioning system. An exhaust
fan vented to the outside of the building is
recommended if there is no separate air-ex-
change system built into the heating and air
conditioning system.
Like all areas of the shelter, the euthanasia
room must be able to withstand frequent
cleaning and disinfecting. The floor of a
euthanasia room is especially vulnerable to
an accumulation of dirt and animal fluids
and waste, and must therefore be completely
sealed; if possible, a central floor drain should
be installed to facilitate drainage. The floor
should also have a durable nonslip surface to
protect employees from falls when handling
animals. All walls, cabinets, and other surfaces
should be sealed with an epoxy-based paint
or covered with a nonporous material for
easy disinfection.
Recommended
Equipment
Euthanizing animals is highly stressful for
humans, physically as well as emotionally.
Physical strain can be caused by lifting, hold-
ing, and restraining animals. A well-equipped
work area that allows for flexibility in handling
and managing animals is therefore essential.
While euthanasia can be performed exclu-
sively on the floor, having a table or other
elevated workspace makes the work much
easier, particularly when euthanizing cats
and small dogs. Ideally, a commercial-grade
grooming or veterinary table will be available
in the euthanasia room, as these typically
can be raised and lowered to ensure that
technicians are working at a comfortable
Euthanasia Tools

49
The Humane Society of the United States Euthanasia Reference Manual
height (a battery powered mobile scissor
lift table is ideal). If a shelter cannot aord
a grooming or veterinary table, any wide,
solid surface that is of a comfortable working
height can be acceptable, provided it has a
nonslip, easily disinfected surface (a stain-
less steel table covered with a rubber mat
to provide secure footing for dogs can be
a good choice).
In addition to the table, there should be a
separate work surface and locking cabinets
for holding syringes, needles, and other
equipment. Assuming the shelter uses a
computerized management system for
recording and updating information regard-
ing individual animals, a computer station
should ideally be available in the euthanasia
room for the technician to verify an animal’s
status and update records throughout the
process. A microchip scanner must be readily
available to scan each animal immediately
before euthanasia.
There should be one or more kennels, large
transport cages, or banks of cages easily
accessible in or near the euthanasia area.
These are necessary to hold animals while
waiting for pre-euthanasia drugs to take
eect, or to securely house animals that
have been administered an IP injection of
sodium pentobarbital.
Few employees can lift a large dog alone
without risking back injuries. Veterinary-
grade stretchers can be useful in transpor-
ting heavy animals when they cannot move
on their own. A flatbed cart or commercial
bin container on wheels is also useful for
removing animals; remember that animal
urine is highly corrosive and will cause ordi-
nary metal or painted surfaces to deteriorate
quickly, so stainless steel is best. No matter
what method of transport is used, each
animal body should be removed from the
euthanasia room before the next conscious
animal is brought in.
Syringes
All syringes commonly used for euthanasia
are plastic and disposable. Most syringes can
be reused, provided that they are adequately
cleaned after each session. However, any
leftover drug that remains in the syringe
can crystallize and cause obstruction when
another drug is introduced. Therefore, it is
recommended that new syringes be used
during every new euthanasia session, and
separate syringes must always be used for
each separate drug administered.
Syringes of 1, 3, 6, and 12 milliliters are most
commonly used with dogs and cats, and
shelters should keep all four sizes in stock.
Larger sizes are available for livestock and
other large animals, and smaller sizes can
prove helpful for rodents and small birds.
For the most part, the larger the syringe, the
more expensive it is and the more unwieldy it
is to use; therefore, technicians should always
select the smallest syringe available to handle
the necessary dose of drug.
Syringes are available either with or without
a locking “hub” for the needle. The hub is
the protrusion at the end of the syringe over
which the needle is fastened. A standard
syringe has a smooth, or “slip,” hub, in which
the needle is simply pushed over the hub and
Human hospitals operate under very
stringent standards regarding the use
and reuse of medical equipment; in some
cases, these protocols prevent them from
using equipment that is actually still in
100 percent working order. While shelters
must be careful to weigh the advantages
and disadvantages of using “secondhand
equipment,” it is never a bad idea to
check with your local hospital or clinic to
see if it has any useful supplies available.
Euthanasia Tools

50
The Humane Society of the United States Euthanasia Reference Manual
held in place by friction. While this type of
syringe is easiest to use, if care is not taken,
the needle may pop o as the plunger pushes
the drug through the small needle opening,
causing the drug to spray into the face and
eyes of the technician. This can be avoided
by ensuring that the hub is dry before
attaching the needle.
A syringe with a hub that allows the
needle to be mechanically screwed onto
the syringe (known as a “locking hub”)
is preferred because it helps prevent the
needle from accidentally coming o the
syringe, an important safety feature.
The locking hub also fixes the place-
ment of the needle on the syringe
at a stationary position.
Most smaller syringes have hubs in the
center of the syringe (these are known as
centric syringes). Larger syringes (more than
6 milliliters) are available with o-center
(eccentric) hubs at the edge of the syringe,
so the needle is on approximately the same
plane as the animal’s leg. This can be a great
advantage because it reduces the angle
between the syringe and the vein, making
a proper injection easier. Unfortunately,
o-center hub syringes are not currently
available in the locking hub style, so care
must be taken when using them to ensure
that the needle does not pop o.
Needles
As noted earlier in this manual, needles
are available in several sizes, called gauges,
according to the bore diameter (the size)
of the needle. The smaller the diameter of
the needle, the larger the gauge size—for
example, a 25-gauge needle is smaller than
a 22-gauge needle, which is smaller than
an 18-gauge needle. Most shelters use 18-
to 25-gauge needles for euthanizing com-
panion animals.
Choosing a smaller needle generally pro-
duces less pain for the animal on injection.
However, smaller needles are more fragile
and more likely to bend with animal move-
ment; moreover, the smaller bore means
that the drug moves more slowly through
the needle, making it impossible to achieve
a bolus eect if a large amount of sodium
pentobarbital needs to be administered. Also,
a small-bore needle on a larger barrel syringe
will create more pressure, potentially causing
the needle to pop o. Therefore, selecting
the right needle for the right animal is crucial.
Typically, cats, puppies, and small animals use
smaller needles for IV injection, 22-gauge and
smaller. Dogs typically do well with 20-gauge
needles, depending on the size of the animal
(although a very small Chihuahua may do
well with a 22-gauge needle). Selecting the
proper needle comes with experience, and is
part of a good euthanasia technician’s toolkit
for making euthanasia as humane as possible
for each animal.
In addition to dierent gauges, needles come
in dierent lengths. A euthanasia technician
will select a short needle for small animals
like guinea pigs or kittens, but will select a
longer needle for larger animals like livestock.
Even if livestock are not common in your
facility, it is important to have longer needles
on hand to facilitate cardiac sticks on deep
barreled or obese animals, in order to verify
death (see Chapter 4). The common needle
Needles and syringes
Euthanasia Tools

51
The Humane Society of the United States Euthanasia Reference Manual
lengths for dog and cat euthanasia are ⅝
inch to 1 inch, and 1½ inch needles are useful
for IC injection.
At a minimum, a brand-new needle should be
used for every animal; best practice calls for
a new needle to be used with every injection.
Reusing needles dulls the tips, and makes
each subsequent injection more painful.
Because veins do not have nerve endings,
the only pain an animal experiences during
a normal IV injection is the penetration of
the skin with the needle, so it is critical to
avoid the unnecessary pain caused by using
a dull needle. Moreover, working with nee-
dles does raise the possibility of the injector
or handler getting accidentally stuck; using
sterile needles helps reduce the risk of
infection afterward. And, as noted above,
never inject an animal with a needle that
has been dulled by insertion through the
seal of a drug bottle.
Sharps Containers
All shelters should use approved medical
waste containers (called sharps containers)
for disposal of used needles. Needles must
never be discarded with general waste
disposal, as they are a potential source
of injury and infection. There is also a risk
that this dirty needle will fall into the hands
of an IV drug user if disposed of improperly.
With the growing concern over medical
waste, some local jurisdictions have strict
regulations about the disposal of needles
and even syringes—in many locations they
must also be disposed of as medical waste
(even if they can be disposed of as general
waste, they should be thoroughly washed
to ensure that leftover drug does not cause
harm). Check with local health authorities
to be sure that your shelter meets all appli-
cable standards.
Tourniquets/Hemostats
While it is recommended that there always be
two people involved in the euthanasia of an
animal, one handler and one euthanasia tech-
nician, there may be times when the handler
cannot successfully “hold o” the vein for the
technician. In those cases, a Nye tourniquet (a
commercially made unit with a metal restrict-
ing device) or a combination of rubber tubing
and hemostat can be clamped just above the
animal’s elbow, constricting the blood flow
in the vein and causing it to enlarge. Once
the vein has been entered, the technician
must be careful to release the pressure before
injecting sodium pentobarbital, as it will
block the drug from smoothly circulating to
the heart. Tourniquets and hemostats can be
bought from veterinary and medical supply
Euthanasia Tools
Recommended Needle
Size/Application
14 gauge, 1 inch/
sodium pentobarbital draw needle
18 gauge, 1 ½ inch/
IC, large dog
20 gauge, 1 inch/
IV, dogs 60 pounds and larger,
IC, small dog
22 gauge, 1 inch/
IV, dog less than 60 pounds
22 gauge, ¾ inch/
IP, adult cat
25 gauge, ⅝ inch/
IV, cats; IP, kittens
Recommended Syringes
Size/Hub Style
1 cc/luer slip or lock
3 cc/luer slip or lock
6 cc/luer slip or lock
12 cc/eccentric luer slip only

52
The Humane Society of the United States Euthanasia Reference Manual
companies; alternatively, shelters can contact
local hospitals and veterinary oces for possi-
ble donations of used equipment.
Scale
The animal’s weight will dictate proper drug
dosage, so having a scale near the euthanasia
room is essential. A walk-on scale is preferred
for adult dogs and cats, and a baby scale works
well for puppies, kittens, small mammals, and
small wildlife. When weighing an adult dog or
cat on a floor scale, if the dog will not stand
long enough to get an accurate weight the
handler may need to weigh himself holding
the dog, then subtract his weight to obtain the
weight of the dog (referred to as tare weight).
If an animal is restrained in a trap or carrier,
the same technique can be used—just subtract
the container’s weight.
Electric Hair Clippers
Electric hair clippers are essential to ensuring
that the technician has the best possible view
of an animal’s leg. Any of the brand-name
grooming or hair clippers is acceptable,
although a cordless commercial-quality model
will provide fewer problems and last much
longer than many of the less expensive mod-
els on the market. A wide range of blades is
available for clippers, but a No. 40 blade is
used most typically because of its close cut.
Follow the manufacturer’s directions for
cleaning and lubricating the clippers, because
without proper care the clippers will burn
out and have to be replaced. A cooling spray
(a specially formulated product) should be
applied to clipper blades every few minutes of
use on the leg of a dog with long or matted
hair to prevent overheating, which can cause
discomfort or burning; use a toothbrush to
remove hair and debris from the blades before
applying the spray.
Other Recommended
Supplies
In addition to the items mentioned above, a
number of items should be present in every
euthanasia room:
• While every shelter should have at least
one comprehensive first aid kit on
the premises, it is advisable to keep an
additional kit in the euthanasia room in
the event of a bite or a scratch.
• An eyewash station approved by the
federal Occupational Safety and Health
Administration is recommended for
accidental exposure to drugs (some states
require this). Alternatively, a manual eye-
flushing device must be easily accessible.
• A high-quality stethoscope is useful for
determining lack of heartbeat (technicians
must be properly trained in using a
stethoscope to verify death).
Euthanasia Tools
Using a tourniquet to hold off the lateral
saphenous vein for injection

53
The Humane Society of the United States Euthanasia Reference Manual
• All animals should be scanned again for
a microchip with a universal microchip
scanner before euthanasia, as a final
opportunity to reunite lost pets with
their owners (remember that metal
tables, fluorescent lights and computers
may interfere with a reader’s ability to
accurately identify a microchip).
• Towels or a rubber pad can be used on
the surface of the table or floor, under
the animal, to minimize slipping and
increase the animal’s comfort; towels can
also be used to darken cages and carriers
for animals that have been given a pre-
euthanasia drug.
• Cleaning and disinfecting supplies,
including paper towels, disinfectant,
squeegee, and plastic bags, should be
easily accessible.
• Drug logbook, calculator, drug dosage
charts, paper, writing pads, etc., are
necessary for compliance with applicable
record-keeping requirements.
• Computer station or laptop can provide
easy access to the shelter’s animal record-
keeping system.
• Animal handling equipment including
nets, control poles, etc. (see Chapter
8) ensure the safety of both animals
and humans.
• Crates, carriers, or caging for animals that
have been administered a pre-euthanasia
drug , to allow the animal to pass through
the stages of anesthesia in a secure and
isolated environment.
Euthanasia Tools

54
The Humane Society of the United States Euthanasia Reference Manual
CHAPTER 7
Euthanasia Policy
and Protocols
No matter how frequently or infrequently
euthanasia is performed in a facility, no other
component of shelter work is as consequential
or will be as carefully scrutinized by the public.
It is imperative that every sheltering facility
have a written euthanasia policy that identi-
fies when euthanasia will be performed, how
it will be performed, and by whom. It should
also provide guidance as to available drugs
and dosages, safety information, restraint
policies, carcass disposal policies, and instruc-
tions on completing all necessary paperwork
and record-keeping to ensure compliance with
state and federal regulations. All sta involved
in euthanasia must be trained thoroughly
on this policy.
Selection Criteria
A facility’s written euthanasia protocol should
provide clear guidance as to which animals
will be considered candidates for euthanasia,
and what the review and approval process for
determinations will be. While these guide-
lines should be clear and consistent, they
must still be flexible; rules that mandate that
every animal be euthanized after a specific
period for time, for example, are arbitrary
and do not serve the best interest of the
animals in the shelter’s care or foster respect
from the public.
A shelter’s euthanasia protocol should specify
that euthanasia determinations are made
by an experienced animal care professional
in a position of authority, preferably in con-
sultation with other key sta members. To
determine whether euthanasia is necessary,
shelters should consider such variables as age,
health, temperament, physical condition,
behavior, and available space, and should
aim to objectively consider other ques-
tions, including:
• What are the realistic prospects for
providing this animal with a good quality
of life?
• Is the animal in pain or distress, and is there
realistic hope of (and financial resources
for) alleviating this pain to allow for a
quality life?
• Does keeping this animal present health or
safety risks to other animals or people?
• Given the fiscal and practical limitations
faced by this organization, does keeping
this animal reduce the organization’s
ability to humanely care for other animals
in need?
• Are there realistic options for rehabil-
itation or management of the animal’s
behavioral issues?
• Is there foster care available for the
animal that will enable the animal to
achieve placement?
• Is there a reputable rescue or alternative
placement available for the animal?
The answers to these questions will vary
greatly depending on the community (and
may even vary by facility in one community)
based on financial and stang resources,
demand for space within the facility, and
other considerations.
Euthanasia Policy and Protocols

55
The Humane Society of the United States Euthanasia Reference Manual
When euthanasia is necessary, typically one
sta member selects the euthanasia candidate
and brings the recommendation to one or more
senior sta members for written approval.
Additional input may be sought from a veteri-
narian or other qualified professional(s). Some
organizations employ committees to make
euthanasia decisions, but for most organiza-
tions that must make these dicult decisions
on a timely basis, a more streamlined procedure
is preferable. To avoid any confusion, the chain
of command for decision-making should be
specified in the policy and strictly followed.
Generally, animals must not be euthanized
before the expiration of their legally mandated
minimum hold time. If an exception must be
made because the animal will endure unnec-
essary suering by being held, the reason for
the exception should be specified in writing,
and, whenever possible, confirmation of the
necessity for the decision should be obtained
by a licensed veterinarian. IMPORTANT NOTE:
Some states mandate veterinary approval
before any animal is euthanized within its
hold period; check with a lawyer or your
state’s oversight authority to ensure that your
process for euthanasia before the expiration
of the legal minimum hold time is lawful in
your jurisdiction.
Although it may be tempting, a policy
requiring 100 percent agreement from
all sta or volunteers, or both, before a
euthanasia decision is reached is dicult
to implement. Not only does it often
result in a stalemate where no actual
decision is reached and the animal sits
in limbo, it forces everyone to carry the
burden of euthanasia-related stress,
even those who are not psychologically
equipped to handle it. A better practice
is to empower individuals to express
their thoughts and concerns, but charge
a few key sta members, those who can
be relied on to make objective decisions
in accordance with policy, to make the
ultimate decision.
Occasionally, stories surface about a shelter
euthanizing the “wrong” animal—either mis-
taking one animal for another (usually because
the animal is in the wrong cage, has the wrong
paperwork, or because he or she is similar in
appearance to another), or mistakenly eutha-
nizing an animal that has a potential adopter or
rescue placement, or worse, an animal that has
an owner. To minimize the chances of such mis-
takes, the protocol should specify steps to verify
that the right animal has been approved and
brought to the euthanasia area before any drugs
are administered. At a minimum, this should
involve checking and cross-checking the animal’s
ID number and description by no less than two
separate people, rescanning the animal for a
microchip, and verifying one final time that there
are no administrative or possible owner/adopter
Euthanasia Policy and Protocols
Determining whether an animal should
be euthanized for behavioral reasons is a
complex process. Evaluation of an ani-
mal’s behavior should begin the minute
the animal arrives in the shelter, and
should continue throughout the stay, as
opposed to being solely dependent on a
“pass/fail” temperament test. A history of
the animal should be solicited from the
owner upon surrender whenever pos-
sible, and daily behavioral observations
should be noted by sta and volunteers.
An objective, formal assessment should
complement this more informal informa-
tion-gathering process, and whenever
possible, shelters should use the results
gleaned to provide additional training
or other behavioral support to help the
animal achieve placement.

56
The Humane Society of the United States Euthanasia Reference Manual
holds associated with the animal. If there are
any doubts, the process should be halted until
all necessary confirmations are made.
It is every euthanasia technician’s worst
nightmare—the wrong animal is euth-
anized. While human error does occur,
the chances of this happening are greatly
reduced if appropriate safeguards are
followed, including:
• All potential owner contact notes
attached to animal’s record.
• Double/triple signature, after review of
dates and records.
• Both handler and technician verify that
it’s the right animal.
• Final scan of animal for microchip
before administration of drugs.
If the worst does happen, and a mistake
is made, acknowledge it, review what
went wrong, and put necessary steps in
place to ensure that the mistake does
not occur again.
Policy Elements
The agency’s written euthanasia policy should
ensure that animals are being euthanized
in accordance with the highest standards of
humane practice, and in conformance with
all applicable state and local laws. Technicians
should be empowered to select the most appro-
priate method of euthanasia for each animal.
The policy should also remind technicians about
the importance of verification of death, and
indicate proper methods for disposal of remains.
Some written euthanasia policies are extremely
detailed, specifying each step of the process,
which drugs are to be used, how drugs are to be
administered, specific animal handling methods,
and more. Others are broader, outlining general
policies and procedures but referring employees
to training manuals, etc., for details. A facility’s
euthanasia policy can be as detailed or general
as desired, but at a minimum it should include:
• Selection criteria for euthanasia.
• Procedure for recommendation and
approval of euthanasia decisions.
• Verification procedures intended to ensure
that the proper animal is euthanized,
including assurances that:
› the proper signatures and approvals have
been obtained.
› the animal’s minimum hold date has
expired or a documented exception
to the minimum hold time has
been obtained.
› the description and identification
numbers of the animal ready for
euthanasia match the approval
paperwork exactly.
› the animal has no adoption, possible
owner or other hold in place.
› the animal has no evidence of ownership
(no tattoo, microchip, or lost report).
• Statement regarding who is permitted
to perform euthanasia (should reflect
applicable training requirements for
the jurisdiction).
• Statement regarding who will be permitted
to be present in the euthanasia room (for
example, will members of the public be
permitted to witness euthanasia?).
• Procedures for performing euthanasia.
• Procedures for verification of death.
• Procedures for proper disposal of remains.
Euthanasia Policy and Protocols
Partnerships with rescue groups and other
facilities can aord lifesaving opportu-
nities for animals that might otherwise
be euthanized. However, facilities must
make rescue decisions responsibly, taking
measures to ensure animals are moving to
a better circumstance.

57
The Humane Society of the United States Euthanasia Reference Manual
• Procedures for drug handling and storage.
• Emergency procedures, sta safety
protocols and applicable personal
protective equipment required.
• Procedures for record-keeping and
administrative compliance (for example,
every facility managing controlled
substances must keep logs detailing the
date of euthanasia, weight and breed
of animal, volume of drugs used, and
technician’s initials, and this log must be
updated as each animal is euthanized).
Volunteers and
Euthanasia
Euthanasia decisions can be a source of tension
between a shelter and its volunteers. Shelter
personnel report feeling that volunteers
who inquire about euthanasia decisions are
overstepping boundaries, while volunteers
feel entitled to basic information about the
outcomes of animals for whom they have
provided care. Clear communication about
expectations and policies is crucial. Volunteers
should be made aware of your organization’s
euthanasia policy from the outset, and they
should be provided a clear channel for commu-
nicating questions about euthanasia decisions
(rather than putting technicians on the spot).
By the same token, while volunteers should not
necessarily be empowered to influence eutha-
nasia decisions, respect for their feelings or
experiences with and knowledge of the animals
they are working with should be considered.
By creating a culture that is open, honest, and
respectful, an organization and its volunteers
can form a successful partnership without strife
over euthanasia decisions.
Euthanasia Policy and Protocols
While shelters are well-served to be
transparent and open about euthanasia
decisions, it is important to be aware of
the eects those decisions have on sta
members. Comments and questions from
employees and volunteers alike (such as
“You’re not going to euthanize that one,
are you?” or “What happened to Fluy?”)
are certainly understandable, but they
can be hurtful to technicians and handlers
already carrying a tremendous burden.
While employees and volunteers should
be given an outlet for their questions and
comments (for example in many shelters
the shelter manager or volunteer coordi-
nator is responsible for addressing such
questions and concerns), they should be
encouraged to respect the feelings of the
sta members involved and refrain from
commenting about euthanasia decisions.

58
The Humane Society of the United States Euthanasia Reference Manual
CHAPTER 8
Animal Handling
and Restraint
Whether the euthanasia technician has
chosen to start the euthanasia process with
a direct injection of sodium pentobarbital or
administration of pre-euthanasia drugs, the
animal must always be humanely restrained.
The handler therefore assumes a crucial
responsibility, ensuring that the animal’s last
interaction with humans is filled with as much
kindness, compassion, and gentleness as pos-
sible while preserving the safety of everyone
in the room. A successful handler must be able
to accurately read the animal’s body language
and behavior, understand how voices and
movements both inside and outside the room
may aect the animal, and be familiar and
comfortable with all the tools available to do
the job most humanely and eectively.
As is the case in virtually every animal han-
dling situation, the least restraint is always
the best restraint. Friendly, well-socialized
animals typically appreciate human contact,
and euthanasia can generally be performed
by gently holding and comforting the ani-
mal while sodium pentobarbital is directly
injected into the vein or abdominal cavity.
Fractious, fearful, or highly aroused animals,
on the other hand, reject human contact,
and become more stressed when forced in
close proximity to people. Proper euthanasia
for these animals requires the least amount
of human interaction and handling possible,
and is typically best achieved via a pre-euth-
anasia drug that allows the animal to lose
consciousness in a dark, quiet, safe enclosure
before a fatal dose of sodium pentobarbital
is administered. No magic formula exists for
determining the best technique; the decision
must be based on the animal involved, his
or her reaction to the situation, and the
resources available. The ability to evaluate
each element is the handler’s greatest asset,
and is crucial to providing the animal a “good
death” with minimal stress and anxiety.
NOTE: All of the following information relates
to the restraint of dogs and cats. Because
of the wide range of variables, it is not
possible to address here all the techniques
that should be applied with other species,
so handlers must consult experts to ensure
that the restraint techniques used are as
humane as possible.
Typical Restraint
Techniques–Dogs
Restraint for Direct Injection
To administer an IV injection to a dog, it is com-
mon practice for the handler to stand alongside
the dog, facing the same side of the dog, and
use the arm closest to the dog’s head to cradle
its neck in the crook of the elbow (he or she can
use that hand to scratch or rub the dog’s ear
or neck as an extra measure of reassurance).
The handler’s other arm then reaches over the
back of the dog and grasps the far front leg just
behind the elbow, extending it for the techni-
cian to inject into. Most well-socialized dogs
will not object to this type of gentle restraint,
but if they do resist, the handler can use the
combined strength of their arm and torso (also
using the rest of the body or even a wall to
serve as a barrier to movement by the animal)
Animal Handling and Restraint

59
The Humane Society of the United States Euthanasia Reference Manual
to help to maximize control. Smaller dogs can
be held in this manner on top of a steady table,
while larger dogs should be allowed to remain
on the floor. Held in this manner, dogs admin-
istered an IV injection quickly fall unconscious
while still in the arms of the handler.
When handling puppies, the handler may
simply draw the puppy up close to his or her
body, using one arm to support the weight of
the body (the puppy’s tail facing the handler’s
elbow, the handler’s fingers between the
front paws supporting the neck and helping
to extend one foreleg) and the other to gently
control the puppy’s head. Alternatively, the
front half of the puppy can be lifted o a table
by a hand slid under his or her chest, expos-
ing the abdomen for IP injection. Whichever
method is chosen, ensuring that the puppy
feels secure is paramount.
Restraint for Administration
of Pre-Euthanasia Drugs
Dogs that cannot be safely restrained with
a moderate amount of handling should be
injected with a pre-euthanasia drug. For mildly
uncooperative dogs, this can be achieved
using the same techniques noted above,
except instead of extending the dog’s front
leg the handler simply uses his or her arm to
help immobilize the entire length of the dog,
allowing the euthanasia technician to safely
hold the animal’s rear leg for an intramuscular
(IM) injection. One eective technique is for
the handler to place his or her arm under the
lower abdomen of the dog, thus helping to
support the dog and using the arm almost as a
barrier against the legs so the dog cannot bend
his or her knee. Alternatively, the dog can be
placed in a press gate, allowing the technician
to safely and quickly reach the dog’s hind legs
for administration of the IM injection (dogs
on control poles can also be gently restrained
for injection, but the use of a press gate is
preferred). Once the injection has been admin-
istered, the dog should be left alone in a quiet,
secure environment until he or she has lost
consciousness, whereupon sodium pentobarbi-
tal can be administered; the handler remaining
with the dog should be prepared to gently
restrain the dog through all the stages of anes-
thesia so the dog does not cause self-injury.
Animal Handling and Restraint
Proper hold of a dog for direct injection
into the cephalic vein
Proper hold of a puppy for euthanasia

60
The Humane Society of the United States Euthanasia Reference Manual
Typical Restraint
Techniques–Cats
Restraint for Direct Injection
Because intraperitoneal (IP) injection, when
performed properly, causes no pain, well-
socialized cats can be injected directly with
sodium pentobarbital with very minimal
restraint. The handler simply places one
hand between the cat’s two front legs,
grasping them gently together, and lifts the
front of the cat slightly o the table surface.
When restrained in this manner, the forelimbs
of the cat need to be lifted only enough to
allow the euthanasia technician to determine
the proper location for injection, and most
cats do not object. Because IP injection takes
time for full eect, after the injection the
cat should be placed in a quiet, dark cage or
carrier until he or she loses consciousness.
IMPORTANT NOTE: If the cat struggles and
resists gentle handling, IP injection should
not proceed; instead, a pre-euthanasia drug
should be administered. Also, cats should
not be stretched for IP injection because
stretching is not only uncomfortable for the
animal, it can result in pain upon injection if
the skin is taut.
Restraint for Administration
of Pre-Euthanasia Drugs
If a cat cannot be safely handled for direct
injection, a pre-euthanasia drug should be
administered. If the cat will accept minimal
handling, simply open the cage door and gently
hold the cat securely in place (either by gently
scrung it, with or without a towel draped over
its head to encourage the cat to calm down
and to provide an extra measure of security, or
by using a clear plastic shield or other device
designed to gently hold the cat in place) with
the head turned away, then inject the cat with
the appropriate pre-euthanasia drug (prefer-
ably Telazol) in the large leg muscle. Once the
cat has been injected, close the cage door and
leave the cat alone or place the cat in another
quiet, dark, secure place to limit stimulation
while the drug takes eect. Once the cat is
unconscious, he or she can be brought to the
euthanasia table and given an IV or IC injection
of sodium pentobarbital. IMPORTANT NOTE:
If pre-euthanasia drugs are administered while
Using both hands to properly support a cat
Mother cats move their kittens by firmly
grasping the nape (scru) of their neck,
with the rest of the body left to dangle.
As cats age, though, they do not appre-
ciate being carried in quite the same
fashion! Therefore, for adult cats the
term “scrung” is said to mean gently
grasping the loose skin on the back of the
cat’s neck, without pinching or twisting
the skin, while at the same time sup-
porting the cat’s weight by cradling the
lower body. If the cat cannot be gently
handled in such a fashion it should not
be considered an appropriate candidate
for IP injection.
Animal Handling and Restraint

61
The Humane Society of the United States Euthanasia Reference Manual
the cat is in a cage, be sure to remove water, so
that the cat does not drown (it is advisable to
remove everything from the cage except the
towel or bedding).
For fractious cats, it is best to avoid any direct
handling. Using a Safety Stick is the best
approach for injecting most unhappy cats—
simply use the stick to gently administer a
pre-euthanasia drug in the large muscle mass
of the leg. Cats that are overtly aggressive
when the Safety Stick is introduced into the
cage will most likely need to be removed
in order to humanely administer the drug
(remember, it is unacceptable to administer
pre-euthanasia drugs in any location besides
a large muscle mass, so a cat that will not hold
still for careful placement of the needle should
not be injected). The cat should be secured in
a net for injection, then returned to a secure,
quiet, dark area to allow the drug time to take
eect. Another alternative is to use the feral
cat box that will hopefully already be in the
cat’s cage—close the box and carry it to the
euthanasia area where IM injection of a pre-
euthanasia drug can be performed through the
holes in the box (although injecting through
the holes in a feral cat box can be challenging).
Alternatively, the handler can either transfer
the cat to a squeeze cage or restrain the cat
with a Plexiglass® shield, net, or “fork” for
an IM injection of pre-euthanasia drugs. Again,
after the injection the cat should be placed
in a dark enclosure to lose consciousness.
General Restraint
Techniques
Regardless of method, everyone involved in
the euthanasia process should speak in calm,
quiet, reassuring tones, and avoid rapid, jerky,
or threatening movements. A calm atmosphere
can relax a nervous animal suciently to avoid
needing additional restraint tools, and can keep
a docile animal tractable.
Even if an animal appears to be receptive to
gentle restraint, a backup plan is crucial in case
something goes wrong. Nets, towels, control
poles, and other tools should be at the ready
in case an animal begins to resist restraint, and
pre-euthanasia drugs should be available to
administer quickly when direct injection is no
longer viable.
When handling rabbits, hamsters, and
other small mammals, the same general
principles apply—the least amount of
restraint is always best. Many mammals
aren’t comfortable being carried, so
keeping them in their familiar enclosures
to the greatest extent possible is best.
And always be sure to support small
mammal bodies fully—never pick up or
carry an animal by the ears or tail, and
be certain that the entire length of their
body is supported.
Restraint Tools
Although there is no substitute for the knowl-
edge and experience of the handler, humane
animal-handling tools are among the most
important pieces of equipment at a shelter.
Standard equipment that should be accessible
at all times includes: leashes, towels, gloves,
control poles, bags, nets, ropes, muzzles, bar-
riers, and various types of confinement units.
When using restraint tools, the principle that
Gentle restraint of a cat for injection
Animal Handling and Restraint

62
The Humane Society of the United States Euthanasia Reference Manual
the least restraint is best always applies—
a control pole or press gate should not be
a first option when the use of a leash or
a towel will do. It is the handler’s responsibility
to read the animal and decide which, if any,
restraint tool(s) should be applied in order
to humanely secure the animal and pre-
serve human safety.
IMPORTANT NOTE: If restraint tools are nec-
essary, use pre-euthanasia drugs instead of
attempting direct injection.
Time
The most crucial, yet most frequently over-
looked, tool for humane animal handling is
time. Animals that appear nervous or fractious
can often be humanely handled with just a
leash or a towel if they are approached slowly
and given sucient time to acclimate to the
handler’s presence. Unfortunately, in shelter
settings, the overwhelming amount of work
to be done and frequent stang shortages
sometimes force sta to rush, even when work-
ing with and around animals. However, it is
vital that handlers appreciate that taking their
time with an animal can avoid a prolonged
battle on a control pole or other device, can
keep all the humans safer, and will save time in
the long run. It is equally critical that those not
involved in the euthanasia process respect this
important point, and ensure that employees
are not interrupted or rushed.
Leash
For dogs, a leash is one of the most basic, and
crucial, pieces of restraint equipment. Provided
the dog has been properly socialized to it, a
leash can help position the animal’s head and
body, and it ensures that the animal will not
bolt away if he or she becomes startled or
frightened. Handlers must always take care
to ensure that the leash does not choke the
dog, as that could cause panic unrelated to the
injection process. A flat cloth or nylon leash
can also serve double duty as a temporary
muzzle when gently wrapped around the
dog’s snout, if no other option is available;
however, a leash wrapped around the dog’s
snout will not provide as much security as an
actual muzzle, and should not be relied on if
there is reason to believe the dog may become
fractious. IMPORTANT NOTE: Leash muzzles
can injure the animal if not applied properly (if
the leash is twisted or pinches the lips), which
can cause the animal to become more dicult
to handle. When possible use a commercially
made muzzle or fashion one out of soft gauze
rather than a leash.
Towel
For most animals, a towel gently draped over
the eyes has a calming eect, which can work
to a handler’s advantage in a euthanasia
setting. Handlers must not simply assume
that a towel will prevent an animal from
biting or scratching—take care to properly
secure every animal whether or not a towel is
used to calm them.
A towel is extremely useful for handling mildly
uncooperative cats. Drop a thick towel over
the cat in a cage so that the head and body
are covered; after allowing him or her to calm
down under the towel, the cat and towel
together can be safely scooped up and carried
from the cage to the euthanasia table. For
slightly less cooperative cats, a towel wrapped
snugly once or twice around the head, legs,
and body will protect the handler from being
scratched. This “kitty burrito” acts much like a
cat bag to minimize scratching and biting, as
the confinement provided by the towel often
calms the cat (this technique can also be used
on small dogs).
For dogs, a towel can even be used as a make-
shift Elizabethan collar, preventing the dog
from turning its head to bite, particularly for
small breed dogs.
Animal Handling and Restraint

63
The Humane Society of the United States Euthanasia Reference Manual
Gloves
Gloves can often provide protection from bites
and scratches, and are an immensely important
tool, particularly when handling a fractious cat
or small animal. But gloves can provide either
thickness and bite protection or dexterity and
sensitivity—not both—making any glove less
than desirable for extensive use in a euthanasia
setting. True animal handling gloves (as opposed
to more traditional general work gloves)
can oer protection from both crushing and
penetrating bites. While general work gloves
(typically leather gloves for construction or
farm work) can be better than nothing, they do
not compare with rugged gloves intended for
animal handling work. Some of the best gloves
available have two layers, using both dense
leather and Kevlar, and may reach all the way
to the shoulder. But typically, the more protec-
tion the glove aords the more restrictive it is.
For that reason, while gloves are useful, many
handlers avoid them unless absolutely necessary.
Gloves should therefore never be considered a
substitute for proper handling technique.
Muzzle
Muzzles are essential for minimizing bite risk,
and should be used whenever there is any
doubt as to whether a dog might become
fractious. Lightweight, easy-to-clean nylon
muzzles, leather muzzles and even metal
basket muzzles are available in a wide range
of sizes and styles. Be certain that any muzzle
is securely applied. Even after application, it
is critical to use safe handling techniques in
the event that the muzzle somehow slips or is
removed by the animal. Once applied, the muz-
zle should not be removed until the animal has
completely lost consciousness, because even
after pre-euthanasia drugs have been admin-
istered the dog may experience an excitement
phase with reduced bite inhibition. A muzzled
dog must be closely monitored to ensure that
he or she does not vomit with the muzzle on,
which could cause the dog to choke; if the
dog vomits, quickly but cautiously remove the
muzzle and allow the dog to expel the vomit.
Dogs panting heavily from stress or exertion
should be allowed to calm down before a
muzzle is placed.
Press Gate
For dogs who can be safely walked on a leash
but may become fractious when IM injection
is attempted, a press gate (also commonly
referred to as a restraint gate or a squeeze
gate) can be a useful alternative to putting the
dog on a control pole. The press gate typically
looks like a small section of chain-link fencing
that has been secured to a wall on one side,
leaving the other side free to swing back and
forth. With the gate swung open, the dog is
walked toward the axed end of the gate;
the loose end of the gate is then gently swung
back toward the wall, securing the dog’s body
between the gate and the wall, allowing the
technician to perform an IM injection in the
dog’s back leg. In most cases, when expe-
rienced personnel use the press gate, the
process runs smoothly; however, there are
occasions where the dog becomes frightened
Animal Handling and Restraint
Application of a muzzle can increase worker safety

64
The Humane Society of the United States Euthanasia Reference Manual
or aroused by the confinement of the gate.
In such cases, the handler can further secure
the dog by running its leash through the front
of the gate and holding it near the floor, to
maintain an extra measure of control over
the front of the dog’s body such that he or
she can’t back out or try to go up and over
the gate. For shelters that have no press gate,
the eect can be simulated using the door
of a traditional dog kennel.
Control Pole
Probably no device has saved more animal
shelter personnel from serious injury than the
control pole (also commonly referred to as a
catch pole, rabies pole, or come-along). When
properly used, the control pole serves, in eect,
as an extension of the handler’s hand, allowing
her to safely secure and guide a dog that could
not be safely approached in closer proximity.
However, the control pole can easily be used
improperly, and can cause serious injury or
even death to a dog. Therefore, a control pole
should be used only when the dog presents a
legitimate risk of serious injury to the handler.
If the dog can be safely walked on a leash, or
the dog is small enough that he or she can be
secured with a towel, a control pole should
not be used. IMPORTANT NOTE: Never use a
control pole on a cat!
Even the most docile cats that are accus-
tomed to wearing a collar will resist a
control pole or other tightening around
their neck. Therefore, control poles
should never be used on cats.
While control poles can be constructed
from simple materials like a pipe and rope,
the quality and construction of commercial
models are far better than any homemade
devices. Several commercially manufactured
types of poles exist, and shelters should keep
them handy in the dog housing areas and
the euthanasia room. Control poles should
be checked frequently to ensure that they
remain in good working order.
As noted above, a control pole should serve as
an extension of the handler’s arm, and should
simply help to guide the dog’s body, not to
choke, yank, or drag it. The noose (loop) of the
pole should be placed as low on the dog’s neck
as possible to avoid crushing the larynx. Apply
just enough pressure on the noose to ensure
that the dog cannot slip out of it—too much
pressure may cause the dog to panic, particu-
larly if the dog feels its airway is being cut o
(a good test of this is to slightly release the
noose on a dog that is struggling on the pole—
if the dog relaxes, it is likely that the noose
was so tight the dog was feeling suocated).
Once the dog is securely on the pole, use it to
guide the animal forward. Never drag a dog
on a control pole; if the dog will not move, the
handler should position herself behind the dog
to encourage him to step forward; the dog
should be permitted to move forward at his
own pace, rather than being pushed with the
A press gate is a useful tool for securing a fractious
dog for injection of pre-euthanasia drugs
Animal Handling and Restraint

65
The Humane Society of the United States Euthanasia Reference Manual
pole. Typically one pole is sucient to secure a
dicult dog; however, for exceptionally large,
fractious dogs, a second pole can be applied by
a second handler to ensure that the handler is
not overpowered. Again, the goal is not to force
a dog to comply through use of one or more
poles, but simply to prevent injury and help
guide the dog in the general direction required.
The condition of the control pole can tell
a great deal about whether it is being
used humanely. If the pole is full of bite
marks, the user may be dragging dogs on
it, putting the pole in range of the teeth,
rather than positioning him or herself
behind the dog and guiding it forward. A
bent or curved control pole suggests that
the user is lifting dogs up with it, which is
an inappropriate use of the tool.
To reduce the anxiety of both the animal
and the handler, the amount of time a dog
spends on the end of a control pole should
be limited. To this end, design of a euthanasia
area and pre-planning for the handling of
aggressive dogs is essential for minimizing
both human and animal stress and to pro-
vide euthanasia safely. Too often, animal
handlers are forced to move an aggressive
dog on the end of a control pole across the
shelter grounds to the isolated euthanasia
area, where by the time they arrive, both
dog and handler are stressed and exhausted.
Instead, if aggressive dogs arrive at the
shelter specifically for euthanasia, they should
ideally be driven directly to the euthanasia
area for a pre-euthanasia anesthetic injec-
tion. Alternatively, they can be placed into
a wheeled transport cage that is brought
directly to the euthanasia room—upon arrival,
the leash or control pole still on the dog
can be pulled taut through the cage door
to restrain the neck of the dog while a pre-
euthanasia injection is given in a hind leg.
While ideally all euthanasia-related procedures
should be performed in a designated euthana-
sia area, for some fractious dogs who become
highly aroused on a pole it may be most humane
to administer pre-euthanasia drugs while still in
the safety of their kennel. Not only is this often
less stressful for the dog, it reduces the chances
that other dogs in the kennel will become
stressed witnessing the dog fight the control
pole. To administer pre-euthanasia drugs to a
dog in a kennel, first secure the area to ensure
that members of the public and volunteers
will not unwittingly stumble upon the scene
and cause a distraction. Next, properly apply
a control pole to the dog and either use the
gate of the kennel as a makeshift press gate or
gently press the dog’s head toward a corner of
the kennel, allowing the technician easy access
to the dog’s hind leg for IM injection. Because
kennels are usually such tight quarters, it will be
critical that the handler is experienced enough
to ensure that the dog remains secure and that
the technician is adept enough to administer
the pre-euthanasia drug eciently and quickly.
Once the drug has been administered and the
technician has safely left the kennel, the dog
should be released from the pole and allowed to
lose consciousness, at which point he or she can
be carried to the euthanasia area on a stretcher
or a cart for injection of sodium pentobarbital.
Squeeze Cage
One of the most practical devices for safely
restraining cats (and even wild animals like rac-
coons, etc.) is a squeeze cage. A squeeze cage
is simply a regular cage or trap with an extra
movable wall inside; this wall can be pushed
or pulled toward the animal until he or she is
unable to move, helping to ensure safe and
humane injection of a pre-euthanasia drug.
Ideally, each shelter should have at least one
commercially manufactured squeeze cage for
use on fractious cats or wildlife. Alternatively, a
makeshift squeeze cage can be made by using
a commercial or homemade “fork” inserted
Animal Handling and Restraint

66
The Humane Society of the United States Euthanasia Reference Manual
through a wire carrier or trap. The fork fits
through the top or side holes of the container
and creates a false “wall” that restricts the
animal to the end of the carrier. A pair of forks
can be used to “walk” the animal into a pressed
position within the cage. The animal can then
easily be given an injection, the fork can be
removed, and the carrier can be covered to
allow the animal to go under the eect of the
drug administered.
Simple clear plastic hand-held barriers can also
provide an eective variation on the squeeze
cage concept. The barrier is inserted into a
regular cage and is used to guide an animal
into a corner, at which point the animal can be
injected through holes in the barrier. When
using a plastic-glass barrier it is critical that it
be properly sized so that the cat cannot escape
over or around it, causing more stress and pos-
sible injury for both the cat and the technician.
Feral Cat Box
Another alternative for humane IM injection of
fractious cats is the use of a commercially man-
ufactured or homemade feral cat box. This is a
small box with small holes on the sides that can
be used for transporting a cat and for IM injec-
tion of pre-euthanasia drugs. A feral cat box is
ideal for use because it can be placed inside the
cage of the cat, oering a safe, stress-free place
for hiding. When the cat is to be euthanized,
simply securely shut the openings of the box
and carry it to the euthanasia room.
Cat Bags/Nets
Cat bags and nets can be very eective for
handling fractious cats. Handlers must be
careful to not just catch the cat in the net, but
also to roll the net suciently to ensure that the
cat is rendered completely immobile, allowing
for safe and accurate IM injection of pre-eutha-
nasia drugs. After the injection, the cat should
be returned to a cage or carrier while the drug
takes eect. While fishing nets can be used to
secure cats, they are typically not intended to
withstand sharp cat claws and they may contain
openings large enough for cats to strike out
with their legs; only nets made specifically for
containment of cats should be used in a shelter.
Cat Graspers/Tongs
Cat graspers/tongs are designed to grasp and
hold a cat securely (the tongs will not tighten
so completely as to injure the animal). They are
very useful for securing and removing cats from
tight spaces where a net cannot be used. They
should not, however, be used for restraining a
cat for euthanasia.
A squeeze cage immobilizes a cat for
injection of pre-euthanasia drugs
Animal Handling and Restraint
A net is a useful tool for securing a fractious
cat for injection of pre-euthanasia drugs

67
The Humane Society of the United States Euthanasia Reference Manual
CHAPTER 9
Employee Health and Safety
NOTE: Nothing in this chapter should
be regarded as medical advice. Always
consult a physician for evaluation or
treatment of any injury.
While participating in euthanasia does pose
inherent risks, as is the case with virtually any
activity involving animals, proper training and
education are vital to help avoid injuries. It
is incumbent on facility managers to provide
the tools, training, and other resources that
sta and volunteers need to minimize risks to
the greatest extent possible.
The tips meant to increase sta safety during
the euthanasia process apply equally well
regardless of the shelter work being per-
formed—working in pairs, for example, not
only helps reduce the chance of problems
(a second pair of eyes is always useful for
spotting trouble before it happens), it also
ensures that someone is there to restrain
the animal and summon help, should a crisis
arise. Having proper, well-kept tools like nets
and control poles is vital to ensuring that
response to incidents is immediate, eective,
and humane. First aid kits and other safety
supplies should be accessible and restocked
as necessary. And last but not least, acknowl-
edging incidents, discussing how they can
be avoided, and adopting changes based on
lessons learned is of utmost importance—if
sta cannot discuss problems because they
fear they will be rebuked or disciplined,
opportunities for prevention of future
incidents are lost.
Bites/Scratches
Even though most injuries, including bites and
scratches, can be avoided through skilled han-
dling, incidents can still occur. If a technician
or handler is injured, follow these procedures:
• Ensure that the animal is immediately
secured and unable to inflict
additional injury.
• Treat the injury as appropriate.
• Report the injury to both shelter
management and public health ocials.
• Ensure that the animal is properly
evaluated for rabies.
• Debrief about what happened, and how
the incident could have been avoided.
• Correct any deficiencies in policies
and procedures.
Sta members may also suer back strain
or other injuries from lifting or restraining
Of course, preventing problems before
they occur is always best—you can reduce
chances of workplace injuries by:
• Having clear policies in place.
• Ensuring that the sta is
properly trained.
• Ensuring that workers have the
time needed to do their jobs
thoroughly and carefully.
• Promoting good communication
and discussion of problems and
opportunities for improvement.
• Ensuring that employees have
all equipment they need, in
good working order.
• Promoting teamwork and a positive,
supportive working environment.
Employee Health and Safety

68
The Humane Society of the United States Euthanasia Reference Manual
animals. Employees should be trained on
proper lifting techniques, and should have
equipment like stretchers available to ease
physical burdens.
Needle Sticks
In addition to sustaining possible injuries
from animal handling, euthanasia technicians
can be injured by accidental needle sticks. If
an animal becomes excited and bumps the
hand holding the needle during injection,
or if the technician becomes careless or
distracted while holding an uncapped nee-
dle, it is possible that a needle will cause a
puncture injury (called a needle stick). If an
accidental needle stick occurs, run the wound
under water immediately to help flush out
any bacteria that may be pushed into the
wound, and cover it, then promptly seek
medical attention.
The primary danger from a needle stick
arises from the puncture itself—the risk
of danger from drugs being injected is
actually quite low. This is not because the
drugs are any less lethal to humans than
they are to animals, but because the quan-
tities necessary to aect a person are
unlikely to be injected accidentally (for an
average-size person, quite a large amount—
greater than a few milliliters—must be
injected to achieve a lethal dose). Typically
when an accidental puncture occurs, only a
minute amount of drug is injected before the
technician reflexively reacts to the pain of
the puncture and removes the needle. Such
incidents usually cause nothing more serious
than some pain or tingling at the injection
site. Nevertheless, always seek immediate
medical attention whenever accidental injec-
tion of even small amounts of drug occurs,
since the possibility of an allergic or other
reaction exists.
The risk of needle injuries can be greatly
reduced by leaving the needle capped until
just before the injection, and by not using
risky shortcuts like pulling the cap o a
needle with the teeth, or worse, trying to
reinsert a needle into a cap held between
the teeth. Using a clean needle each time will
help reduce the risk of infection if a needle
stick does occur. But perhaps most important
to avoiding accidental needle stick injuries is
clear, eective communication between tech-
nician and handler to identify and prevent
potentially risky situations.
Eye Injuries
Sodium pentobarbital and pre-euthanasia
drugs can cause significant injury if they
are sprayed into a person’s eyes. This most
typically occurs when the needle pops o
the syringe during injection, either because
the needle was not properly secured or
because the needle became clogged and
could not withstand the pressure applied
during injection. When any drug comes into
contact with an eye, the situation should
be considered a medical emergency. The
eye immediately should be flushed volumi-
nously with water (which is why it is advisable
to have an emergency eyewash station in
the euthanasia room). Contact lenses should
be removed immediately, and first aid pro-
cedures for this type of injury should be
started at once.
The use of locking syringes, the selection
of appropriately sized needles, and the
steady administration of pressure can help
prevent needles from accidentally popping
o, and using clean needles every time
can help avoid blockage. Nevertheless,
the only way to definitively avoid any risk
of accidental eye injury is by requiring sta
to wear protective eyewear.
Employee Health and Safety

69
The Humane Society of the United States Euthanasia Reference Manual
General Hazards
Most environmental injuries are the result of
inadequate safety measures, vague proce-
dures, or human error. Accidents that result
from the first two causes may be reduced by
providing proper training, solid procedures,
and a good selection of high-quality restraint
equipment; human error, unfortunately, is
impossible to completely eliminate. Because
of the nature of the work, the euthanasia
area is the site of many potential work haz-
ards, like slippery fluids or hair clippings on
the floor, cage doors left open, and the like.
A good way to minimize injuries from these
sources is to provide sta with adequate
time to accomplish their tasks eectively
and thoroughly.
Compassion Fatigue/
Euthanasia-Related
Stress
Animal shelter workers and volunteers must
contend with a unique paradox in their lives.
They enter the field of sheltering because
of their love for animals and their desire to
care for those that find themselves victims
of homelessness, or worse. Yet the work of
caring for animal victims exposes them to a
phenomenon called “compassion fatigue,” a
form of secondary post-traumatic stress dis-
order in which caretakers begin to internalize
and suer the eects of the trauma experi-
enced by the victims they care for. And while
most suerers of traumatic stress experience
a trauma and are able to move through the
stages of grief to eventually heal, for caretak-
ers the grief process begins anew with every
new animal victim that comes through the
door—there is never any final resolution or
psychological healing.
To complicate matters further, euthana-
sia technicians must suer the additional
psychological burden of being the only
caretakers who often are called upon to end
the lives of the very victims for whom they
have provided care. This dilemma has been
referred to as “caring-killing paradox,” and it
dramatically aects the lives of every eutha-
nasia technician.
Those suering from compassion fatigue and
euthanasia-related stress describe symptoms
that can include: disruption of daily activities,
lack of concentration, sleep problems, feel-
ings of inadequacy and lack of self-worth,
family conflict, substance abuse, depression,
burnout, and negative job satisfaction. The
stresses of the job are typically manifested in
one of two ways—internalizing (exhibiting
self-destructive behaviors like smoking or
For worker safety, a euthanasia room should
ideally contain an eye wash station
Employee Health and Safety

70
The Humane Society of the United States Euthanasia Reference Manual
drinking, avoiding social situations, or oth-
erwise ceasing activities that once provided
pleasure and personal satisfaction) or exter-
nalizing (sudden outbursts of anger, open
hostility toward others, etc.). While both
are normal, neither is productive. Instead,
employees must be supported in finding ways
of managing their grief without harming
themselves or others.
Every shelter must acknowledge that com-
passion fatigue and euthanasia-related stress
are real, and must support their employees’
and volunteers’ psychological needs. Both
employees and volunteers must be made
aware of the signs and symptoms so that
they can better understand and actively
support one another and help build coping
skills. Many organizations have taken steps
to provide support for sta and volunteers,
with internal support groups or professional
counselors to help build coping skills, and still
others use agency-provided employee-assis-
tance programs. Organizations can also take
internal policy measures intended to combat
compassion fatigue and euthanasia-related
stress, including rotating euthanasia respon-
sibilities among a number of sta members,
allowing technicians to transfer the task to
another technician if a day is going particu-
larly badly, allowing technicians to participate
in all of the positive aspects of shelter work
(like reuniting animals with their owners
and facilitating adoptions, instead of only
performing euthanasia). It is also critical that
shelter leadership create a culture that views
euthanasia decisions as an operational neces-
sity of the organization as a whole, rather
than as decisions made by a few individuals;
a culture that allows just a few individuals to
bear full responsibility is likely to generate
high amounts of sta dissatisfaction, burn-
out, and turnover.
Employee Health and Safety

71
The Humane Society of the United States Euthanasia Reference Manual
CHAPTER 10
Federal Requirements for
Controlled Substances
Any agency using sodium pentobarbital or
other controlled substances must adhere to
the strictures of the Controlled Substance
Act (part of the Comprehensive Drug Abuse
Prevention and Control Act), passed in 1970
and enforced by the U.S. Drug Enforcement
Administration (DEA). This law classifies drugs
into five major categories (called Schedules)
according to their abuse potential, and strictly
regulates their manufacture, distribution, and
handling according to their potential risks and
medical benefits.
Under federal law, animal shelters are
not expressly permitted to obtain or use
controlled drugs like sodium pentobar-
bital (a Schedule II controlled substance).
Most shelters obtain euthanasia drugs in
one of three ways:
1. They may hire a sta veterinarian who
can obtain drugs pursuant to their
professional license. This is the most
ideal circumstance, but unfortunately
many agencies do not have veteri-
narians on sta.
2. They may team with an o-site veter-
inarian willing to provide drugs to the
agency; in such cases, the veterinarian
assumes all responsibility for ordering
and obtaining the drugs, administer-
ing their use, ensuring their security,
overseeing inventory, and reporting
any losses or theft to the DEA. This
collaboration works well for many
organizations, although in some more
remote areas there is no local veteri-
narian willing or able to oversee the
shelter’s use of euthanasia drugs.
3. They may take advantage of a state law
that enables direct licensing of shel-
ters, allowing them to obtain and use
drugs without the direct oversight of a
veterinarian. Assuming that the shel-
ter’s euthanasia technicians have been
properly trained, this is the best option
for shelters without a sta veterinarian,
(although it is highly recommended that
the agency still maintain a close working
relationship with at least one expert in
shelter medicine to assist in developing
and overseeing euthanasia practices).
Federal Requirements for Controlled Substances
Controlled Drug Schedules
Schedule I: drugs that have a high
potential for abuse and no currently
acceptable medical use.
Schedule II: drugs that have a high potential
for abuse but have a currently acceptable
medical use (sodium pentobarbital).
Schedule III: drugs that have less potential
for abuse and have a currently acceptable
medical use (Telazol, ketamine, sodium
pentobarbital mixes).
Schedule IV: drugs that have a low
potential for abuse and have a currently
acceptable medical use.
Schedule V: drugs that have the lowest
potential for abuse and have a currently
acceptable medical use.
For the full list of controlled substances and their class-
ifications please refer to: www.deadiversion.usdoj.gov/
schedules/orangebook/c_cs_alpha.pdf

72
The Humane Society of the United States Euthanasia Reference Manual
All shelters that use euthanasia drugs must
meet federal requirements for the storage,
record-keeping, inventory, and disposal of
controlled substances, as well as any applicable
state laws and regulations, which may be even
more stringent than the federal requirements.
For specific information about your state’s laws,
contact the appropriate regulatory agency,
like the state board of pharmacy, board of
veterinary medicine, or state animal control or
humane association. Bear in mind that when
more than one legal requirement applies (for
instance, if there are both federal and state
requirements relating to drug storage), the
agency must meet or exceed whichever version
is more strict.
Storage
Federal law specifies that all controlled
substances be stored in a “substantially
constructed,” securely locked cabinet. The
law does not define exactly what constitutes
“substantially constructed,” so organizations
must look to the intent of the provision for
guidance—since the intent of the require-
ment is to deter theft or misuse of drugs, they
should be stored in a cabinet that is either
permanently constructed or attached to a
building structure to prevent physical removal.
The following are suggestions to ensure safe
and lawful storage of drugs; agencies should
check with their local DEA oce for more
specific recommendations.
• Store the central supply of controlled
substances (the unopened, sealed bottles)
in a floor safe (cemented into the floor), a
safe securely bolted to the floor, or a safe
weighing more than 750 pounds. Store the
daily supply (opened bottles) of controlled
substances in a double-locked steel cabinet
bolted to the wall.
Know Your Laws
• What are the minimum hold times
animals must be held before adoption,
transfer, or euthanasia? Are there
any exceptions?
• Does your jurisdiction permit you
to apply to obtain, possess, and use
euthanasia drugs directly or only with
veterinary authority and oversight?
• What are the training and
certification requirements for
performing euthanasia?
• What drugs are permitted? (For
example, some states prohibit the
use of sodium pentobarbital in
powder form; other states dictate
which pre-euthanasia drugs may
or may not be used.)
• What administration methods
are permitted? (For example, some
states prohibit oral administration
of sodium pentobarbital.)
• Are there other laws that may aect how
euthanasia is performed? (For example,
in some states mixing of ketamine and
xylazine is prohibited as a violation of
pharmacy compounding laws.)
A wall-mounted drug safe for storing controlled substances
Federal Requirements for Controlled Substances

73
The Humane Society of the United States Euthanasia Reference Manual
• Because hydrated (mixed) Telazol should
be kept refrigerated, a lock box should be
mounted in the refrigerator itself.
• If state regulations allow controlled
substances to be carried in a vehicle,
such supplies should be kept in a locked
box that is securely fixed to the inside of
the vehicle.
• Access to the drug supply should be
limited to supervisors, veterinarians,
and properly trained and certified
euthanasia technicians.
• For high-volume shelters with a large
supply of controlled substances
kept on hand, a security alarm or
surveillance system should be installed
as additional protection.
• A master drug inventory log recording
every time the supply safe is opened
should be maintained to ensure that
the inventory remains accurate (this log
should be separate and distinct from
the euthanasia log used to record each
euthanasia performed, but the individual
bottle numbers, etc., should be capable
of being cross-matched). A witness should
be present and the drugs counted every
time a sta member opens the safe, and
the log should be updated with each new
shipment received or bottle removed for
use in the shelter. The log should include:
a) The drug’s shipment lot number and
manufacturer/distributor name.
b) The drug type and name.
c) The in-house assigned bottle numbers
(used to match specific bottles with
specific animals on the euthanasia log).
d) The drug’s strength, volume, and
expiration date.
e) The date and amount of drug (number
bottles in consecutive order) received.
f) The date and amount of drug (number
bottles in consecutive order) removed.
When more than one controlled substance is
used at a facility, each controlled substance
must have its own section within the logbook.
It is good practice to track each drug used in
the euthanasia process, even those that are
not considered to be controlled substances
(like xylazine).
Record-Keeping
Requirements
A detailed log recording the exact use of
controlled substances for euthanizing individ-
ual animals is also required to be maintained
by shelters. These records should be kept in a
bound logbook with numbered pages rather
than in anything with detachable pages, like a
loose-leaf binder. The following information
must be included in the euthanasia log:
• The type of drug(s) used.
• The in-house number assigned to the
bottle(s) from which the drugs were drawn.
• The name of the person administering
the drug.
• The description (species, breed) of each
animal euthanized.
• Each animal’s identification number.
• The weight of the animal euthanized.
• The amount of each drug used.
• The total amount of the drug remaining
in each drug bottle after use (this record
should demonstrate that 100 percent of
the drug from each bottle was used).
Sample page from a euthanasia logbook
Federal Requirements for Controlled Substances

74
The Humane Society of the United States Euthanasia Reference Manual
Inventory Records
Federal law requires that a full, detailed inven-
tory of all controlled substances be performed
at least every two years (individual states
may impose more stringent requirements).
However, it is recommended that shelters
inventory their controlled substances much
more frequently, ideally as often as monthly.
This constant close monitoring helps keep
the inventory process simple, and it helps
to promptly account for any discrepancies
between the records and the shelter supply of
controlled substances.
Shelters must maintain inventory and other
records for controlled substances on site for a
minimum of two years, and these must be kept
separately from ordinary business records. All
records, including those kept on computer files,
must be easily readable and retrievable for DEA
inspection on demand.
Penalties for failing to comply with state
and federal drug laws can range from
minor fines to possible criminal charges,
depending on the nature of the infraction.
Reporting Theft/Loss
Federal law requires that the loss or theft of
any controlled substance be reported to a DEA
field oce immediately. Disposal of outdated
or unwanted controlled substances must follow
strict DEA requirements.
Training of Euthanasia
Technicians
There are no specific federal regulations
regarding the training of shelter personnel
using controlled substances; however most
states have specific training and certification
requirements in place. For specifics about
certification requirements for your state
contact the appropriate regulatory agency,
like the state board of pharmacy or board of
veterinary medicine, or state animal control or
humane association.
Federal Requirements for Controlled Substances

75
The Humane Society of the United States Euthanasia Reference Manual
CHAPTER 11
Unacceptable Methods
of Euthanasia
The following methods of killing animals are
not considered to be humane euthanasia, and
should never be permitted in a shelter setting:
If you have been ordered to perform
an inhumane practice, do not com-
ply, and do not remain silent! Alert
an ocial with regulatory power over
the facility (like a veterinary board, the
Department of Agriculture, etc.), national
animal advocacy organizations like The
Humane Society of the United States,
and if necessary alert your local media.
Every individual euthanasia technician is
responsible for not just understanding
and using humane euthanasia practices,
but for stopping inhumane practices from
being performed by others.
• Injection of neuromuscular blocking
agents (succinylcholine chloride, T-61,
strychnine, curare, etc.) which when
administered to a conscious animal
(or human), induce muscle paralysis
and respiratory distress without loss
of consciousness. In eect, with these
drugs the animal is rendered completely
unable to move, to breathe, even to blink,
even though the animal is fully awake
and aware, and able to experience pain,
fear, and panic; this can go on for several
minutes until the animal eventually
succumbs to respiratory paralysis.
• Carbon monoxide (CO) (gas chamber).
• Electrocution.
• Drowning.
• Cervical dislocation.
• Carbon dioxide (CO2).
• Decompression.
• Pithing.
• Decapitation.
• Exsanguination.
• Ether.
• Air embolism.
• Nitrogen flushing.
• Injection of acetone or any other solvent.
• Injection of Roccal-D or any
other disinfectant.
• Injection of sedatives like chloral hydrate.
• Injection of any agent other than sodium
pentobarbital, xylazine, ketamine, ACE,
Telazol, or other approved drug (including
caeine, nicotine, magnesium sulfate/
epsom salts, potassium chloride, chloral
hydrate, etc.).
• Any combination of sodium pentobarbital
with a neuromuscular blocking agent.
• Any combination of the above.
IMPORTANT NOTE: Gunshot should never be
used in the shelter environment; there should
always be a more humane alternative available.
Unacceptable Methods of Euthanasia

76
The Humane Society of the United States Euthanasia Reference Manual
CHAPTER 12
Euthanasia of Other Animals
Many organizations care for animals other than
dogs and cats, and at times these animals must
be euthanized. Ensuring that this process is as
humane as possible for each animal, regardless
of species, is just as critical as it is for dogs and
cats. NOTE: These species-specific guidelines
are intended to complement the basic eutha-
nasia information provided above; they are not
intended to serve as a substitute for thorough
knowledge of, experience with, and training
regarding the behavior and physical character-
istics of each species. Euthanasia technicians
should not try to handle species with which
they are not familiar and comfortable.
Small Mammals
All small mammals have sharp teeth and may
bite when startled or in pain; they are also
capable of moving very quickly, and can injure
themselves or even become lost in the shelter
if not handled properly. Care must be taken to
ensure that each individual is handled and euth-
anized humanely. Whenever handling small
animals for euthanasia, the technician should
try to make them feel as physically secure and
comfortable as possible—use towels to securely
wrap rabbits, for example, and “scoop” smaller
animals like mice in the palm of the hand. Never
grab small animals by the tail or the ears, and
ensure that their bodies are fully supported
at all times. Gloves can be useful to help avoid
bites that break the skin, but they are not a
substitute for calm, careful handling.
Rabbits
Although rabbits should be euthanized with
sodium pentobarbital in the same manner
described earlier for cats and dogs, because
of their high metabolism they require fully
twice the amount of drugs. For IV injection,
therefore, at least 2 milliliters of sodium
pentobarbital should be used per 10 pounds
of animal, and for IP injection 6 milliliters is
the minimum recommended. Both PreMix
and Telazol are acceptable pre-euthanasia
drugs for rabbits. Rabbits should never be
euthanized using a gas chamber or another
inhalant agent.
Small Rodents (Mice, Rats,
Hamsters, Gerbils, etc.)
Because it is nearly impossible to inject into
the veins of most small rodents like mice, rats,
gerbils, and hamsters, the most eective mode
of administering euthanasia drugs is by IP
injection. Gently grasp the animal’s back and
neck from the top—for some small animals the
technician can gently “scru” the nape of the
neck while holding the animal in the palm—and
inject into the midline. If the animal cannot
Euthanasia of Other Animals
A squeeze cage is useful for securing wildlife
for injection of pre-euthanasia drugs

77
The Humane Society of the United States Euthanasia Reference Manual
be gently and securely handled, or if handling
would cause the animal undue stress, the
technician should either administer a pre-eu-
thanasia anesthetic (Telazol or PreMix) or use an
inhalant anesthetic like halothane to render the
animal unconscious before injecting the drug IP.
Guinea Pigs
Guinea pigs should be euthanized with sodium
pentobarbital in the same manner described
previously for cats and dogs, typically using
IP injection because of their tiny veins. Both
PreMix and Telazol are acceptable pre-euthana-
sia drugs (guinea pigs can actually pass out from
fear or stress, so administration of pre-euthana-
sia drugs may be advisable). Guinea pigs should
never be euthanized using a gas chamber or
other inhalant agent.
Ferrets
Like cats, ferrets react quickly in their attempts
at escape from unfamiliar handling, and cau-
tion should be used when handling even tame
ferrets. Ferrets can be restrained for euthanasia
either by wrapping them securely in a towel or
by scrung the body for IP injection, similar
to a cat. If the ferret can be handled, direct IP
administration of sodium pentobarbital is the
most eective means of euthanasia. If not,
either PreMix or Telazol are acceptable pre-
euthanasia anesthetics.
Birds
Birds are generally more susceptible to stress
than any other group of animals, and even
small birds like parakeets, canaries, or other
songbirds can deliver painful bites. Regardless
of the bird’s type or size, be careful to ensure
that stress levels are reduced as much as
possible both for the animal’s comfort
and human safety.
Small birds can generally be handled using
a soft washcloth or towel; gently cover the
bird, then grasp gently from behind, placing
the bird’s head between the first and second
fingers and cupping the bird’s body in the palm
of the hand. Chickens and other larger birds
can be restrained using larger towels to wrap
and restrain the head and wings. Gloves and
eye goggles are highly recommended when
working with any of the larger bird species, like
fish-eating birds or raptors, as both their sharp
beaks and their talons may inflict serious injury,
and it is very common for a bird in distress to
stab out at a person’s eyes.
Extra caution must be taken when han-
dling birds used for fighting, as they may
have sharpened spurs or razor attachments
that could easily injure or even kill a person.
Small birds can be anesthetized before
euthanasia using an inhalant anesthetic like
halothane; larger birds should be administered
an injection of PreMix or Telazol into the
muscle mass overlying the breastbone or at
the back of the thigh. Once the bird is uncon-
scious, injection of sodium pentobarbital can
proceed. Even though birds do not have a true
peritoneal cavity like mammals, sodium pen-
tobarbital can be injected into the body cavity
directly below the keel, or breastbone, perpen-
dicular to the body. Alternatively, IV injection
can be performed directly into the veins
Euthanasia of Other Animals
Proper site for injection of bird

78
The Humane Society of the United States Euthanasia Reference Manual
running along the wings. For technicians expe-
rienced with feeding birds, oral administration
of sodium pentobarbital may be yet another
option (assuming oral administration is per-
mitted by law); using a curved dosing needle
the drug can be simply squirted into the bird’s
mouth, but care must be taken to ensure that
the drug is not accidentally introduced into the
trachea where it will cause suocation.
Very large birds like raptors and ratites
(ostriches and emus) are dangerous, and
euthanasia should not be attempted without
skilled assistance.
Reptiles
Reptiles have physiological dierences that
pose specific challenges for euthanasia. Reptiles
in general have a much slower metabolic rate
than either mammals or birds, requiring a
longer time for euthanasia drugs to take eect.
Moreover, many of these animals can appear
to be lifeless while just lying dormant, making
verification of death extremely dicult. For
these reasons, it is particularly important that
technicians not attempt euthanasia of reptiles
unless they have considerable experience with
the species in question.
Technically speaking, most reptiles’ breathing
and heart rates can be monitored in much the
same way as one would monitor a mammal
or bird. Respirations can be seen as the chest
and abdomen move in and out, and the heart
may be seen beating if the animal (particularly
lizards and snakes) is examined from the bot-
tom. However, all reptiles can survive with an
extremely low heart rate and respiratory rate,
especially if the body is chilled. Mistakes can
be made, therefore, if the technician relies on
visual observation alone to make a conclusion
about death. Moreover, while heart sticks are
technically possible, they can be very dicult
to perform on reptiles, particularly when the
animal has a plastron (shell) that blocks access
to the heart. For these animals, best practice
is to leave the body at room temperature
overnight (whenever relying on rigor mortis
to verify death it is best to cover the animal
with a towel). Within 12 hours, the eyes should
be dull and sunken, and the limbs, head, and
neck should be limp (or extremely sti, if the
cycle of rigor mortis is incomplete); if this isn’t
the case, the animal may not actually be dead,
and additional sodium pentobarbital should
be administered.
All reptiles can be euthanized using a two-
step process: 1) administer PreMix or Telazol
at a dosage of four times that recommended
for dogs and cats (2 milliliters for every
10 pounds, instead of 0.5 milliliters per 10
pounds), then return them to their enclosure
until they become limp; 2) administer sodium
pentobarbital either through IC, IV or the
abdominal cavity.
Be sure to always wear gloves when han-
dling reptiles and wash hands thoroughly
after, as they can be carriers of salmonella
and other pathogens.
Snakes
Although most snakes are harmless, any snake
that cannot readily be identified should be
regarded as potentially dangerous. Some
snakes can injure or kill people with their
venom or constricting ability, so only an expert
and experienced handler should handle large
or poisonous snakes. A plastic or heavy-wire
screen barrier may be used to approach and
capture nonpoisonous snakes, and specifically
designed snake-handling tools (hooks and
gloves) should be used to increase handler
safety. Appropriate restraint involves control
of the head (grasp immediately behind head)
plus adequate support of the body (as a general
rule there should be one handler for every five
feet of snake).
Euthanasia of Other Animals
79
The Humane Society of the United States Euthanasia Reference Manual
Snakes can be euthanized either by direct
injection or with the use of a pre-euthanasia
anesthetic. If using Telazol or PreMix as a
pre-euthanasia anesthetic, inject into the
muscle along the backbone. Once the snake
is unconscious, an IC injection of sodium
pentobarbital may be given; the heart of
a snake can be located by visually observ-
ing the heartbeat in between the first and
second thirds of the underside of the body.
Alternatively, IV injection into the ventral
(tail) vein is an option. Direct injection
into the coelomic cavity (body cavity space
similar to IP cavity) can also be performed.
Whenever injecting snakes be sure to use
a small gauge needle and inject under the
animal’s scales, do not try to push the needle
through the scales.
Turtles, Tortoises,
and Terrapins
All turtles, tortoises, and terrapins (all collec-
tively referred to in this section as turtles) can
bite and strike with surprising speed, and some
species, like snapping turtles, are dangerous
and can inflict serious injury. Turtles can also be
dicult to restrain because of their strength
and protective shell.
To inject a turtle it is necessary for the head
and limbs to be extended. This can pose quite
a challenge, particularly because some species
can actually draw their head and legs fully into
their shells and then close the hinged shells
completely over these appendages. Trying to
force a limb out of a shell will only increase the
animal’s stress level, and may result in a painful
bite. Better practice is to simply place the animal
on flat surface until the head and tail reemerge,
then quickly grasp a limb before it can be
retracted again; most hard-shell species cannot
hinge their shells closed if a limb is extended.
To safely work with a front leg, use a towel or
other soft cloth to gently press the turtle’s head
to the side and hold it safely out of biting range;
for larger species, the head can be restrained
by gently placing an object like a coee can or
a toilet plunger over their heads and pressing
it in toward the shell.
Crocodilians (Alligators
and Crocodiles)
The jaws, feet, and tail of crocodilians are
extremely dangerous. Only experienced
handlers should try to restrain a crocodilian
that has not been chemically restrained, so
professional expertise from qualified zoo or
wildlife personnel will be needed. If PreMix
or Telazol is to be used for pre-euthanasia
anesthesia it should be administered via a pole
syringe into the muscle mass at the base of the
tail or top of the rear leg. Once the animal is
unconscious, an IV injection into the jugular or
ventral (bottom) tail vein is preferred. NOTE: If
the animal is over four feet long, and must be
euthanized outside the shelter environment,
safety may dictate euthanasia by gunshot
rather than by injection.
Lizards
Larger lizards are potentially dangerous
because of their sharp claws and teeth
and strong tails. In addition, a few species
are venomous. It is prudent to always wear
A pre-euthanasia anesthetic can be
injected into the rear leg of a turtle
Euthanasia of Other Animals

80
The Humane Society of the United States Euthanasia Reference Manual
gloves when handling lizards. Appropriate
restraint involves control of the head (grasp the
neck area above the shoulders and behind the
jaw) and the tail (grasp the base of the tail, right
next to the body, for most eective control).
Pre-euthanasia anesthetic is preferred to mini-
mize the stress of handling and to increase the
safety of the handler. Following unconscious-
ness, an IV injection into the jugular or ventral
(bottom) tail vein may be given. An IC injection
may be made directly into the heart only if
the animal is unconscious. The heart can be
accessed from the underside of the lizard’s body
and is found between the front and middle
thirds of the body. The heart may be located
by visualizing its beating, with a stethoscope,
or by feeling the pulse with a finger or the
palm of the hand.
Fish
To euthanize fish in the shelter setting, it is
most practical to dissolve 6 milliliters of sodium
pentobarbital into a quart of water and intro-
duce the fish to be euthanized; death will occur
quickly, although verification of death in fish
can be challenging (awaiting rigor mortis is
recommended). Alternatively, a commercial
fish anesthetic like tricaine methanesulfonate
(commercially sold as FinQuel) can be used, at
overdose levels.
Amphibians
Amphibians (toads, frogs) can be dicult
to handle because of the slippery protective
mucous layer coating their skin. Many
amphibians also have skin glands, generally
toward the back and to the side of the
head, which exude a toxic substance under
stress. Latex gloves are recommended for
any sta handling amphibians. The advan-
tage of amphibian skin, however, is that it
is actually very absorbent, so euthanasia
can be achieved by simply allowing the
animal to absorb sodium pentobarbital
placed directly on its body (3 milliliters
for every 10 pounds).
Alternatively, euthanasia can be achieved
by injecting ketamine directly into the
thigh muscle. The animal should be kept
in a warm, dark, quiet place while waiting
for the drug to take eect, then adminis-
tered an IP injection of sodium pento-
barbital through the ventral midline.
Large Domestic
Mammals
Large mammals (horses, cows, etc.) have
the potential to inflict grave injury if they
are improperly handled. Most people
know that horses, for example, can both
bite and kick, but euthanasia technicians
must also be aware that certain drugs can
increase the chances that the animal will
react violently. Moreover, large animals
injected with euthanasia drugs can drop
to the ground quickly, and anyone caught
in the path of a falling half-ton animal
is liable to be seriously injured. For these
reasons, unless the euthanasia technician
and handlers are experienced both in
handling large animals and euthanizing
them, they should defer to the expertise
of a large animal veterinarian.
Euthanasia of Other Animals
The notion that fish, reptiles, and amphib-
ians can be euthanized simply by placing
them in a freezer is false. The AVMA’s
Guidelines on Euthanasia state:
Immobilization of reptiles by cool-
ing is considered inappropriate and
inhumane even if combined with
other physical or chemical methods of
euthanasia. Formation of ice crystals
on the skin and in tissues of an animal
may cause pain and distress.

81
The Humane Society of the United States Euthanasia Reference Manual
Equines (Horses,
Donkeys, Mules)
Equines can be euthanized by direct injection
of sodium pentobarbital into the jugular
vein. However, because of the large quan-
tities of drug required to achieve euthanasia
(technically speaking a 1,000-pound horse
requires 100 milliliters of sodium pentobar-
bital, although the manufacturer indicates
that there is a “dose ceiling” of about 60
milliliters, after which little further eect
is really achieved), the animal is likely to
fall to the ground well before the full dos-
age of drug is administered, creating a situa-
tion that is potentially dangerous for the
technician and handlers. A safer practice
is to administer pre-euthanasia drugs to
the animal; the drugs will take several
minutes to achieve full eect and cause
the animal to fall to the ground, increasing
the odds that all of the people involved will
be safely out of the way.
When handling an equine for euthanasia
it is important to remember that the head
of an equine is very heavy, and can cause
serious injury when the animal falls; it is
vital that at least one handler holds a lead
rope on the animal’s halter, and that he is
prepared to help guide the animal’s head
to the ground when it falls, rather than
just allowing the head to move unsecured.
A common approach is to turn the head
toward the animal’s left (or “near”) side
(the one from which the handler traditionally
mounts or leads the horse) and help push
the horse down and back, away from the
handler, as the horse starts to drop.
Administered alone, xylazine can actually
increase an equine’s propensity to kick;
however, when combined with another
drug like acepromazine or ketamine it can
be a very eective pre-euthanasia anes-
thetic for equines.
Ruminants (Cows,
Goats, Sheep)
Cows and other split-hooved ruminants like
goats and sheep are very sensitive to xylazine,
making it the preference for pre-euthana-
sia anesthesia. Alternatively, if the animal is
securely restrained in a chute or if it is small
enough to be held (and the confinement will
not increase the animal’s stress) direct injection
of sodium pentobarbital into the jugular vein
can be performed.
Pigs
Pre-euthanasia anesthetic is needed for most
pigs since they are dicult to restrain and their
veins are very deep and dicult to find. Pigs are
particularly sensitive to Telazol, so that is the
best option to render the animal unconscious
before administration of sodium pentobarbital.
If a technician is experienced, he or she may
be able to administer an IV dose (1 milliliter for
every 10 pounds of body weight) of sodium
pentobarbital into the large vein that is clearly
visible in a pig’s ear.
Wildlife
Unlike the majority of domesticated animals,
wild animals are easily stressed and threatened
by contact with humans, whether that contact
is direct or indirect; just the presence of people
nearby causes tremendous stress. This fact not
only makes it virtually impossible to achieve
Euthanasia of Other Animals
The perceived elevated threat posed by
handling wild animals is often used to
justify the use of less humane euthanasia
methods like gas chambers. However,
if technicians are well trained in safe
handling techniques there is no reason
that traditional EBI cannot be used for all
wildlife species.

82
The Humane Society of the United States Euthanasia Reference Manual
a humane death for such animals, it elevates
concerns about employee safety. However, this
is not an excuse to practice less-than-humane
methods; instead, technicians must work that
much harder to make the process as safe and
stress-free as possible.
In the shelter setting, the environment in
which wildlife is held before euthanasia is
of critical importance. The goal should be to
provide as stress-free a setting for the animal
as possible. To facilitate this, consider the
following recommendations:
• Isolate as much as possible from the
sights and sounds of domestic animals.
• Choose a location away from high-
trac areas.
• Restrict sta access to holding areas or
places where wild animals might be held.
• If possible, dim the lights in the area.
• Maintain as quiet a setting as possible.
• Restrict the animal’s ability to see
what is going on around him or her
by covering his or her container with
towels or blankets.
• Perform the euthanasia as quickly
as possible.
Contrary to popular belief, North
American porcupines are not able to
project their quills. They, however, can
flip their tails and slap the quills into
anything the tail touches.
The principles of euthanasia of wild mammals
are the same as for cats and dogs, as described
previously. However, administration of pre-
euthanasia drugs resulting in unconsciousness
should be considered mandatory to alleviate
the animal’s stress as quickly as possible. Oral
administration of sodium pentobarbital is an
option if the animal is already in a trap or
cage, and if his or her stress level can be
reduced suciently that he or she will eat;
mix the drug in canned dog or cat food, care-
fully place it within the animal’s reach, and
leave the vicinity until the animal has lost
consciousness. If the animal is too stressed or
injured to eat, direct IM injection of pre-eutha-
nasia drugs will be required. To restrain the
animal for administration of pre-euthanasia
drugs, ensure that he or she is safely confined
in a cage, carrier, or trap, and use a trap fork,
heavy blanket, or other method to safely press
the animal into position for IM injection into a
muscle mass. Control poles should not be used
on wild animals unless the loop of the pole is
placed around one of the animal’s front legs
and the neck to prevent strangulation.
Skunks are capable of projecting spray
up to 13 feet. Cover their cage/trap with
a towel and move as slowly as possible
to limit their stress and reduce their
chances of spraying.
Bats
Bats in particular should be considered poten-
tially dangerous because they bite readily and
are a primary carrier of rabies. If possible, avoid
direct handling of bats by using nets and other
restraint devices. If the bat must be handled,
wear leather gloves and use a thick cloth or
towel to restrain the wings and legs.
A technician can use an inhalant anesthetic like
halothane to render the animal unconscious,
followed by an IC dose of sodium pentobarbital.
Alternatively, the technician can immobilize the
bat in a net, grasp the bat gently but firmly with
gloved hands, and administer an IP injection
with a very small-gauge needle.
Euthanasia of Other Animals

83
The Humane Society of the United States Euthanasia Reference Manual
Deer, Elk, and Other
Large Hooved Animals
Extreme caution must be exercised when
handling large hooved animals, since most
adult deer, elk, moose, and the like are capable
of breaking a human’s leg or inflicting other
grave injury with a single kick. Antlers can
also be used eectively as weapons. Males will
be particularly excitable and likely to behave
aggressively during the fall breeding season.
Administration of a pre-euthanasia drug
like PreMix will be required for such animals,
followed by injection of sodium pentobarbital.
Alternatively, outside the shelter environ-
ment the animal may have to be humanely
euthanized by gunshot if drugs cannot be
safely administered.
Bears, Coyotes, Mountain
Lions, Primates, and
Other Large Mammals
Even if large carnivores are not native to your
community, there is the potential that a captive
animal (being housed in a small zoo or by a
private owner) may escape and be injured by
a passing car or otherwise require euthanasia.
Contact your local state or federal wildlife
authority to assess which animals are likely to
be housed in your area (based on local permits
issued, etc.) and to develop a plan for handling
potential incidents.
While a good recommendation for all
animal care workers, pre-exposure rabies
vaccinations should be a requirement for
anyone who may come into contact with
rabies-vector species, including euthana-
sia technicians.
Euthanasia of Other Animals

84
The Humane Society of the United States Euthanasia Reference Manual
CHAPTER 13
Field Euthanasia
In most cases, animals picked up by an ocer
or humane agent are transported to a shelter
facility or veterinary oce. However, some
situations may make transport impossible and
require that euthanasia be performed on site
(this is commonly referred to as “field euthana-
sia”). This most often occurs when the animal
is so gravely ill or injured that he or she is likely
to suer a painful death during transport, the
ill or injured animal is so large that he or she
cannot be reasonably transported even to a
nearby facility, or there are other extenuating
circumstances that make transport of individual
animals unreasonable (for example, when a
truck full of livestock is involved in a trac acci-
dent and dozens of mortally wounded animals
are spread out along the roadway). Regardless
of the circumstances, it is critical that every
animal receives a humane death.
Special care must be taken when field
euthanasia of ill or injured domestic
animals is required, particularly when they
are wearing a collar with identification
tags. The facility’s field euthanasia policy
must be expressly clear about any and all
procedures required, including documen-
tation to be provided, approvals that must
be obtained, witness statements that must
be taken, etc., to avoid potential liability.
Just as is the case with in-shelter euthanasia,
field euthanasia must follow policies and pro-
cedures established by each organization. Field
euthanasia policies should include, but not be
limited to, guidance regarding:
• What approvals will be necessary to
authorize field euthanasia. (Will a
veterinary recommendation be required
for all animals or only for companion
animals like cats and dogs?)
• What state and local laws apply to
emergency euthanasia situations.
• Whether euthanasia drugs are permitted to
be taken out of the shelter facility, and if so
how they will be stored and managed.
• What types of firearms will be permitted for
field euthanasia, and how they will be used.
• What methods of euthanasia will
be permitted.
• What steps must be taken to minimize
agency liability.
• What notifications, if any, of local law
enforcement must be made (particularly
if firearms are to be discharged).
• How should the presence of members
of the public aect euthanasia decisions
and methods.
Assuming all policy issues have been addressed
and field euthanasia will proceed, the ocer
must determine the appropriate method
of euthanasia. A number of factors must be
considered, including the type of animal(s)
involved, the condition of the animal(s), the
potential risks involved to the ocer and to
public safety, and the public perception of
the method selected. To complicate matters,
these situations invariably occur under dicult
conditions—in dark of night, bad weather,
and sometimes even in dangerous locations.
Nevertheless, ensuring the most humane eutha-
nasia possible for the animal(s) must always be
of paramount importance.
Typically, in a field situation an ocer must
choose between euthanasia with sodium pen-
tobarbital and gunshot (gunshot is appropriate
Field Euthanasia

85
The Humane Society of the United States Euthanasia Reference Manual
only in field situations, and should never be
used in a shelter environment). Technically
speaking, either method is potentially available
for any animal; however, EBI is often preferred
for smaller animals where the ocer can safely
approach the animal, and gunshot is typically
preferred for larger animals and situations
where direct handling is not possible.
Stories abound of law enforcement pro-
fessionals shooting dogs because they
believe their safety is at risk. A well-run
animal control agency must ensure that
local law enforcement can trust them to
manage situations involving animals with
non-lethal means.
Confinement for
Field Euthanasia
Regardless of the euthanasia method, the
animal must first be suciently controlled.
Obviously, injection of euthanasia drugs requires
direct handling; however, even gunshot requires
precise targeting to ensure instantaneous death.
In many cases, the animal will already be recum-
bent or have its movement restricted. If not,
the animal will need to be humanely confined
and restrained in order to carry out safe and
eective euthanasia. The ocer must therefore
have a variety of humane confinement methods
available, including blankets, nets, traps, ropes,
halters, and even chemical immobilization meth-
ods like injection poles and darts.
It is always best to try to steer the animal into
a pen or enclosure that allows him to remain
on his feet but prevents significant move-
ment. For smaller animals, cages and carriers
are typically used; for larger animals, boards,
fencing, and gates may be used to create a
makeshift enclosure.
If confinement is not an option, the ocer will
have to use a rope noose or plastic-coated cable
wire to contain the animal; horses, cattle, sheep,
and other livestock can have makeshift halters
placed over their neck and head to control their
forward and backward motion; for pigs, place
the noose over their upper jaw, behind their
canine teeth. Remember, animals are generally
much more powerful than humans, and animals
in pain and fear can be especially dangerous.
Wild animals like deer pose extra challenges in
that even the smallest can inflict grave injury.
Ocers must take extreme precautions to keep
themselves and the public safe when trying
to confine or restrain any animal, and should
never attempt to do so without the help of a
trained assistant.
Field Euthanasia by
Injection of Sodium
Pentobarbital
Assuming the animal has been safely secured,
the ideal means of euthanasia even in field set-
tings is injection of sodium pentobarbital after
administration of a pre-euthanasia drug, as
described in this manual. Any ocer perform-
ing field injection must take extra precautions
to ensure that the animal is properly and
safely handled, as the additional stress of the
uncontrolled field environment combined with
whatever trauma the animal has suered will
cause many animals to react violently.
Some state laws prohibit euthanasia drugs
from being carried in vehicles or require direct
Field Euthanasia
Bear in mind that the conditions in which
the animal is found can influence which
method of euthanasia is most appropri-
ate; for example, it can be exceedingly
dicult to inject into the veins of an
animal that has been exposed to extreme
cold. Technicians in the field must there-
fore be well-versed in all of the humane
options potentially available.

86
The Humane Society of the United States Euthanasia Reference Manual
supervision of such drugs by a licensed veteri-
narian when o shelter property. Ocers
should check with their state ocials to find
out the legal requirements for their jurisdic-
tions. If drugs can be carried, maintaining the
same security for controlled substances in the
vehicle as in the shelter is crucial. Bolting a
combination or keyed metal lock box to the
floor of the vehicle behind or under the seat,
out of sight, provides security for carrying
a small amount of controlled substances.
Procedures generally recommended are
to maintain the supply of controlled drugs
in a safe at the shelter, check out the necessary
drugs at the beginning of the shift, and then
log and return the drugs at the end of the
shift. No controlled substances should ever
be left in an unattended vehicle overnight.
Field Euthanasia
by Gunshot
While euthanasia by injection is preferred, it
sometimes is simply not an option (for example,
if an animal is so large and fractious that trying
chemical capture is impossible). In such limited
cases, a properly trained ocer may elect to
dispatch the animal by gunshot, provided he or
she is: a) trained and certified in the use of the
weapon; b) certain that the discharge of the
firearm will pose no threat to the public; and
c) confident in hitting the exact spot necessary
to ensure that the animal dies instantaneously
without enduring further suering.
Types of Firearms
Rifles
Because rifles can drive a bullet at a greater
velocity than other weapons, significantly
increasing the risk of ricochet and injury
to onlookers, their use for field euthanasia
is not recommended. A rifle should be used
only as a last resort in cases where an injured
animal cannot be approached, but where
an expert marksman can nevertheless
target the exact spot necessary for a
humane death.
Handguns
General-purpose handguns can be used to
kill animals humanely, provided: a) the muzzle
of the gun must never be placed in direct
contact with the animal’s head—instead,
the ocer should shoot from a distance of
approximately two inches and aim down
the length of the neck into the main bulk of
the body; and b) the ocer must use round-
nose lead bullets to facilitate proper
penetration—the use of target-shooting
ammunition is unacceptable.
Shotguns
Used properly, shotguns are the best choice
for field euthanasia by gunshot because they
are much safer than handguns or rifles and
are equally eective. For all species, a 12-, 16-,
or 20-bore shotgun may be used with number
4, 5, or 6 birdshot. A 28-bore or a .410 can be
used if nothing larger is available, but should
not be used on mature bulls or large pigs.
As with handguns, the muzzle should never
No ocer should attempt field euthanasia
without all appropriate certifications and
training. Field euthanasia tests the skills of
the most experienced ocers, and should
not be attempted unless the ocer is
confident that it will be achieved safely
and humanely. If in doubt, the ocer
should call in a professional better able to
manage the situation, be it a more experi-
enced ocer, a veterinarian, or even a law
enforcement ocer with more experience
handling such high stress circumstances.
Calling in “backup” is not a sign of weak-
ness or failure—it is recognition that
such situations must be handled with
the utmost care and professionalism.
Field Euthanasia

87
The Humane Society of the United States Euthanasia Reference Manual
be placed in direct contact with the animal’s
head; instead, the muzzle should be held 2 to
10 inches from the animal’s forehead, aim-
ing down the line of the neck into the main
bulk of the body.
Ordinary-sized birdshot is capable of inflicting
significant injuries when it initially strikes the
skull as one compact mass, giving it consid-
erable initial penetrating power. Once inside
the skull, the pellets will separate and disperse
within the brain cavity, eectively destroying
the brain and killing the animal. As such,
a shotgun is a much safer and usually more
readily available firearm than is a weapon
using a single bullet.
IMPORTANT NOTE: Particularly when using
a free-bullet weapon, it is important that a
suitable backstop be found to block the bullet
if it exits the body or if the target is missed.
Suitable backstops include manure heaps,
hay or straw stacks, and earthen banks. There
should be no “dead ground” (hidden dips)
between the target and the backstop from
which people, vehicles, or other animals could
suddenly appear. If no backstop is available, the
area behind the target must be clear of roads
and dwellings for a distance of approximately
3,000 yards. (A bullet from a .32-caliber hand-
gun can ricochet and travel in excess of 2,000
yards.) In such a circumstance, everyone nearby
must stand behind the ocer, who should
aim the shot down the spine and into the
body of the animal.
In order to be considered humane euthanasia,
a gunshot must cause death instantaneously.
To do this, the bullet must destroy the brain
stem, the portion of the brain that controls vital
functions like breathing, achieving loss of pain
and loss of consciousness at the same moment
it causes loss of life. Hitting the brain stem
requires precise positioning of the bullet, and
this position varies by species; therefore, o-
cers must be thoroughly trained on the precise
location required to eect humane euthanasia
by gunshot for each type of animal they may
encounter in the field. IMPORTANT NOTE: All
firearms must be discharged at close range into
the brain. The muzzle of the firearm, however,
should never come in contact with the animal’s
head; such placement could result in a burst
gun barrel and severe injury to the ocer.
When an animal is properly shot, it will col-
lapse immediately and stop breathing, and
all vocalizations will cease. It will also exhibit
physical reactions that may be disconcerting to
the untrained observer: the animal will bleed
profusely from the entry wound, mouth, or
Ocers should assume that any ill
animal they encounter may have been
exposed to rabies, and should handle
them with extreme caution. They should
also be mindful that because rabies
testing requires the dissection of the
brain, if the animal must be submitted
for rabies testing it should not be eutha-
nized by gunshot.
Any ocer that carries firearms as
a possible means of euthanasia must
strictly observe all general firearms
safety rules, including:
• All firearms must be handled as if
they were loaded at all times.
• Ocers must never engage in behavior
that may result in an accidental dis-
charge while holding a firearm, such
as climbing fences, etc.
• Firearms must never be carried in a
pocket or waistband; a pistol case or
gun holster with a safety flap or strap
must always be used.
• No firearm, loaded or unloaded,
should ever be pointed at anything
not intended as a target.
• A loaded firearm should never be left
unattended or carried in a vehicle.
Field Euthanasia

88
The Humane Society of the United States Euthanasia Reference Manual
nose (a thick plastic bag can be used to prevent
blood from pooling); there may be exagger-
ated contraction of the muscles; the eyes will
assume a fixed, glazed expression; the body
may start to twitch and in some cases convulse
quite violently (particularly in the case of pigs)
for a minute or longer. While disturbing to see,
these are all to be expected even if the animal
has been successfully euthanized by gunshot. To
confirm that the animal is in fact dead, the o-
cer should verify the lack of rhythmic breathing
and lack of blink reflex; if in doubt, the animal
should be promptly shot a second time.
Correct Shot Location
by Species
Cattle
A cow’s brain is situated high in the head. The
ideal point of penetration is in the middle of
the forehead, at the crossing point of two
imaginary lines drawn from the middle of
each eye to the base of the opposite horn. This
should put the target about two inches above
a line drawn across the forehead at the back of
the eyes. The shot should enter at a right angle
(90 degrees) to the skull. For a calf, the upper
portion of the brain is not yet developed, so
the firearm should be aimed at a point slightly
lower than that in the adult animal, and it
should be tilted back to obtain the correct
angle for the shot to destroy the brain stem.
IMPORTANT NOTE: While the target location
remains the same, mature bulls may have a
hard, thick frontal bone, often covered in
dense, matted hair, which can be dicult to
penetrate with a small-caliber weapon; for
these animals a shotgun is a better option.
Deer
A deer’s brain is situated high in the head.
When determining the ideal aiming point,
know that the antlers are not comparable to
the horns on cattle. Instead, the ideal aiming
point is in the middle of the forehead, at the
crossing point of two imaginary lines drawn
from the middle of each eye to the top of
the opposite ear. In stags, this spot is found
between the antlers. The angle of the shot
should be through the brain stem, as in cattle.
Horses and Other Equines
A horse’s brain is situated high in the head.
The shot should be aimed in the middle of the
forehead, but slightly higher than the aiming
point in cattle. Two imaginary lines should be
drawn from the middle of each eye to the base
of the opposite ear; the aiming point should be
approximately three-quarters of an inch above
the point where they cross. The muzzle of the
firearm should be tilted slightly upward or
downward so that the shot is directed through
the cerebral cortex toward the brain stem. If a
horse is holding his or her head in a lower than
normal position, care should be taken to adjust
the angle of the shot.
Sheep and Goats
For sheep and goats, the aiming point is in
the midline, just above the eyes, with the shot
directed down the line of the spine and into the
bulk of the body. This can be dicult to achieve.
A slight error in the angle of the shot or small
movement by the animal can result in a free
bullet exiting the animal’s throat or neck. To
prevent this, the animal’s head must be in the
normal position before shooting.
Heavily horned sheep and goats can present a
problem. The mass of horn can leave little or
no target area. A shot between the eyes is too
low and should never be used. Such animals can
be shot from behind the poll (the top of the
head), but it can be dangerous to do so with a
free-bullet weapon and the animal must always
be on soft ground. Whenever possible, a shot-
gun is recommended for this type of shot.
Pigs
Pigs are among the most dicult animals to
shoot. The target area is very small, and some
pigs are “dish-faced” because of age or breed
Field Euthanasia

89
The Humane Society of the United States Euthanasia Reference Manual
characteristics. The brain lies quite deep in
the head with a mass of sinuses between the
frontal bone and brain cavity. The ideal site for
shooting pigs is one finger’s width above eye
level, on the midline of the forehead, aiming
toward the tail.
Older pigs and exotic breeds, like the
Vietnamese pot-bellied pig, often have
a deep, bony mass on the forehead, which
can cause problems when using a free-bullet
weapon. The bullet can become lodged in
the sinuses and fail to penetrate the brain.
Some older pigs, especially boars, have a ridge
running down the center of the forehead.
In such cases the muzzle of the gun should
be placed slightly to one side of the ridge
aiming into the center of the head. Because
of the problems that might occur with adult
and exotic-breed pigs, a shotgun should be
used if possible. The shooting position is the
same with a shotgun. The animal can also be
shot from behind the ear aiming toward the
middle of the head.
Disposal
After the euthanasia, arrangements must be
made to have the animal’s body removed
from the scene. Small animals can be trans-
ported to the shelter for routine disposal.
Larger bodies, however, may need special
accommodations, like a rendering company.
IMPORTANT NOTE: If a large animal like a
horse is euthanized in a confined space, like
a stall or pen, the body should be removed
from the enclosure as soon as possible, since
the onset of rigor mortis will make it very
dicult to move later. If a body is to be buried,
it must be at least 250 yards from any well,
borehole, or spring that supplies water for
human consumption, and at least 30 yards
away from any other spring or watercourse.
Some jurisdictions prevent the burial of
animal carcasses, so ensure that the means
of disposal selected is lawful.
Angles and points of entry for correct shot locations
for horses, cattle, goats, pigs and sheep
Field Euthanasia
HSI

90
The Humane Society of the United States Euthanasia Reference Manual
CHAPTER 14
Mass Euthanasia
While a written policy will ideally lay out proper
procedures for virtually every euthanasia that
must be performed in the shelter, there may be
cases where it is necessary to euthanize large
numbers of animals o-site, either because of
their physical condition (for example, several
equines in such poor condition that they cannot
be humanely transported) or because of the
sheer number of animals involved (for exam-
ple, large-scale hoarding cases). In such cases,
it is still critical to ensure that every animal is
treated humanely.
Typically, in cases where immediate euthanasia
o shelter property is necessary to end extreme
suering, the lead veterinarian on the scene,
in consultation with the shelter ocial in
charge, will determine which animals must be
euthanized and what manner of euthanasia is
most appropriate. Depending on the type and
numbers of animals involved, euthanasia by
injection may not be practical or safe; nev-
ertheless, the number of animals involved or
complexity of the situation should not excuse
inhumane practices. IMPORTANT NOTE: Even in
cases of extreme suering, minimum hold peri-
ods and other legalities may still apply; it is vital
to ensure that all applicable legal mandates are
followed, particularly if the animals are being
seized as evidence of cruelty or another crime.
Removing euthanasia drugs from the shelter
facility may be subject to regulation—be certain
to obtain all necessary legal authorizations
before taking drugs o site. Typically the
veterinarian on scene will be responsible for
providing and overseeing the use of all drugs,
and must ensure that proper record-keeping
requirements are met.
If carcass disposal will be managed through the
shelter’s normal crematory or landfill service, it
may be necessary to call for an extra pickup. If
disposal must be managed through a rendering
company or other service, arrangements should
be made as quickly as possible. In no case
should carcasses simply be left behind.
Depending on the nature of a mass euthanasia
event and the number and type of animals
involved, psychological debriefing of sta
may be prudent. Shelter management should
arrange for such debriefing within one to three
days, and should determine whether sta
attendance will be mandatory. Even without a
formal debriefing, any sta member involved
in a mass euthanasia event should be allowed
to request psychological assistance at any
time, and arrangements to fulfill such requests
should be made promptly.
Supplies needed for o-site mass
euthanasia typically include:
• Syringes appropriate for species.
• Needles.
• Appropriate drugs.
• Stethoscope.
• Towels.
• Sharps containers.
• Humane handling equipment.
• Clippers.
• Tourniquets/hemostats.
• Plastic garbage bags.
• Log sheets.
Mass Euthanasia

91
The Humane Society of the United States Euthanasia Reference Manual
Glossary
Analgesia: Drugs that have an analgesic eect
are intended to diminish an animal’s ability
to perceive pain, although not all drugs have
the ability to extinguish pain completely. An
analgesic eect does not mean that a drug will
cause unconsciousness in an animal.
Anesthetic: When an anesthetic agent has
been administered at proper dosages, the ani-
mal is ideally rendered unconscious, has a total
loss of ability to feel pain (analgesia), and is
immobilized, yet her vital functions (breathing
and heartbeat) are retained. For this reason, the
ideal pre-euthanasia drugs are anesthetics.
Aspiration of Blood: A means of verifying
that a needle is properly in the vein, by which
the technician pulls back on the plunger
and ensures that there is a flow of blood
into the syringe.
Blink (Palpebral) Reflex: A method used to
confirm lack of consciousness, by which the
technician gently touches the inside corner
of the animal’s eye; if no automatic blink
reflex occurs, the animal can be presumed to
be unconscious.
Cephalic Veins: Veins running prominently
down the front leg of most animals.
Consciousness: When conscious, an animal
has the ability to deliberately and intentionally
respond to environmental stimuli.
Euthanasia: From the ancient Greek—eu +
thanatos, meaning “good death”.
Handler: The person holding the animal
for euthanasia.
Heart stick: Placing a needle into the heart
muscle to verify death (acceptable only when
the animal is unconscious).
Hitting the Vein: Successfully inserting a nee-
dle into the vein to accept injection of drugs.
Holding O the Vein: Using one’s hands or
a mechanical device (such as a tourniquet) to
restrict the flow of blood from the vein to the
heart, making the vein easier to find for pur-
poses of injection.
IC: Intracardiac—injection of drug into the
heart (acceptable only when the animal
is unconscious).
IM: Intramuscular—injection into the muscle
(not a permissible route of administration for
sodium pentobarbital).
Immobilization: When immobilized, the
animal is essentially paralyzed and unable to
move, but he may still be aware of his surround-
ings, may still feel pain, and may actually be
experiencing fear and panic. For this reason,
immobilizing agents are never appropriate for
use in euthanasia.
IP: Intraperitoneal—injection of drug into the
abdominal [peritoneal] cavity, the gap between
the organs and the abdominal wall.
IV: Intravenous—injection of drug
directly in a vein.
Jugular Vein: Veins running down each side
of the neck (not appropriate for euthanasia of
dogs and cats).
Label Dose: The actual required dosage of
sodium pentobarbital that should be admin-
istered for euthanasia, per label instructions.
The label dose is higher than the lethal dose,
so it provides a safety cushion to ensure that
if the proper amount of drug is administered
in the proper manner, the animal will in
fact die humanely.
Glossary

92
The Humane Society of the United States Euthanasia Reference Manual
Lateral Saphenous Veins: Veins running down
the outside rear leg of the animal, crossing diag-
onally across the leg just above the hock.
Lethal Dose: The minimum amount of drug
sucient to move the animal through all four
stages of anesthesia and stop the core functions
of life (respiration and circulation). Technicians
should administer the label dose of sodium pen-
tobarbital, not the lethal dose, to ensure that
sucient quantities of drug are administered.
Medial Saphenous Veins (aka femoral veins):
Veins running down the center of the inside of
the animal’s rear legs.
PO: “Per Os” meaning “by mouth”, oral admin-
istration of drug by squirting directly into the
mouth or mixing into food.
Raising the Vein: Making the vein easier to
spot for injection through pumping the foot,
applying water, etc.
Sedation: When sedated, an animal falls into
a sleep-like state and becomes uncoordinated,
with relaxed and unresponsive muscles. There
is often a decreased ability to feel pain, but
pain sensations are still possible. Sedated ani-
mals may appear to be sleeping but may quickly
become aroused when stimulated by light or
sound, and could cause harm to themselves
and the humans around them.
SQ: Subcutaneous—injection under the skin
(not a permissible route of administration for
sodium pentobarbital).
Technician: The person administering
euthanasia drugs.
Toe Pinch Reflex: A method to confirm lack of
consciousness. The technician firmly pinches
the webbing between the toes of the animal;
if there is no reflexive pull of the leg away
from the pain, the animal can be presumed to
be unconscious.
Tranquilization: When tranquilized, the animal
usually is calm, relaxed, and may even fall
asleep. The animal may still feel pain, however,
and a tranquilizer may not oer enough of
a calming eect to safely handle a fractious
animal. Tranquilized animals may also suer
seizures, and can be more unpredictable.
Unconsciousness: When rendered uncon-
scious, the animal lacks awareness and the
capacity for sensory perception, appearing
to be in a deep sleep.
Units of Measure:
cc = cubic centimeter (measure of
volume/space)
ml = milliliter; one 1000th of a liter
(measure of liquid volume)
mg = milligram; one 1000th of a gram
(measure of weight/mass)
1 cc is generally equivalent to 1 ml
Glossary

93
The Humane Society of the United States Euthanasia Reference Manual
Dosage Chart for Telazol
®
and PreMix
(ketamine/xylazine combination)
Animal’s Weight (pounds) Milliliters (ml)
5 0.25
10 0.5
15 .75
20 1
25 1.25
30 1.5
35 1.75
40 2
45 2.25
50 2.5
55 2.75
60 3
65 3.25
70 3.5
75 3.75
80 4
85 4.25
90 4.5
95 4.75
100 (and up) 5
(.5 ml/10 pounds)
94
The Humane Society of the United States Euthanasia Reference Manual
IV—Intravenous (Injection of sodium pentobarbital into a vein)
• Species recommended: dogs, calm and friendly cats
• Dose: 39 mg/pound (usually 1 ml/10 pounds)
• Circulatory compromised: 78 mg/pound (2 ml/10 pounds)
• Injection speed: rapid (1–2 ml/second) and consistent with good technique
• Dog veins: cephalic, lateral saphenous
• Cat veins: cephalic, medial saphenous (femoral)
• Syringe retracts: small volume (flash) of blood
• Time to loss of consciousness: ~5 seconds
• Time to deep anesthesia: ~10 seconds
• Time to cessation of respiration: ~20 seconds
• Time to cessation of heartbeat (death): ~40 seconds
• Time to cardiac standstill (no fibrillation): ~2–5 minutes
IC—Intracardiac (Injection of sodium pentobarbital into one of the four heart chambers)
• Unconscious animals only
• Species recommended: all
• Dose: 39 mg/pound (usually 1 ml/10 pounds)
• Circulatory compromised: 39 mg/pound (usually 1 ml/10 pounds)
• Needle insertion point: right or left side, 4th intercostal space, or sternally in cats
• Syringe retracts: large volume (fill) of blood
• Injection speed: slow (0.5–1 ml/second) and consistent with good technique
• Time to cessation of heartbeat (death): 5–10 seconds
• Time to cardiac standstill (no fibrillation): ~2–5 minutes
IP—Intraperitoneal (Injection of sodium pentobarbital into the abdominal cavity—
needle passes through the skin, muscle wall and peritoneum)
• Species recommended: conscious calm and friendly cats, conscious puppies under
5 weeks, conscious or unconscious small rodents
• Dose: 117 mg/pound (usually 3 ml/10 pounds)
• Circulatory compromised: not usually recommended
• Injection speed: slow (0.5–1 ml /second) and consistent with good technique
• Needle insertion point: ventral, midline, ~2" below (caudal to) umbilicus
• Needle angle/depth: right angle to the skin/~3/4 inch
• Syringe retracts: suction (no fluid, no air)
• Time to loss of consciousness: ~2 minutes
• Time to deep anesthesia: 3–4 minutes
• Time to cessation of respiration: ~6 minutes
• Time to cessation of heartbeat (death): ~8 minutes
• Time to cardiac standstill (no fibrillation): ~10 minutes
Injection Methods—Quick Reference

95
The Humane Society of the United States Euthanasia Reference Manual
Index
Index
A
Accidental injections, human, 51, 68
Acepromazine, 35–36, 75, 81
Alligators, 79
American Veterinary Medical Association
euthanasia criteria, 1–3, 80
Amphibians, 80
Analgesia, 3
Anesthesia
stages of, 4–7
Animal bites, 67
Animal-related injuries, 49, 67–69
Animal handling and restraint, 58–65
Aspiration of blood, 17–18
B
Bats, 46, 82
Bears, 83
Birds, 77–78
Birdshot, 86–87
Bites, 67
Blink reflex, 27–28, 41, 88
Blown veins, 21–22
Brain
anatomy of, 6
gunshot eects, 87–89
sodium pentobarbital eects, 4–7, 19,
26, 41, 43
C
Carbon dioxide, 75
Carbon monoxide, 1, 75
Carcass disposal, 45-46, 89, 90
Cardiac stick, 41–44, 50
Cat bags/nets, 66
Cat graspers/tongs, 66
Cats
injection diculties, 8, 10, 24
internal organs view, 21
pre-euthanasia anesthetic dosages, 33–37
recommended needle size, 51
restraint of, 20, 60–61, 66
sodium pentobarbital dosage, 22
Cattle
euthanasia procedure, 81
restraint of, 85
shot location for field euthanasia, 88–89
Central nervous system, 4, 35–36,
eect of acepromazine, 35
eect of sodium pentobarbital, 4
eect of xylazine, 36
Cephalic vein injections, 9–13, 18
Cerebral cortex
gunshot eects, 88
sodium pentobarbital eects, 5
Cerebrum, 5–6
Chloroform, 39
Consciousness, 3
Controlled substances
classification of 32, 71
controlled drug schedules, 71
storage of, 72–73
record-keeping requirements, 73
inventory records, 74
reporting theft/loss, 74
Control poles, 64–65
Coyotes, 83
Cremation, 45–46
Crocodiles, 79

96
The Humane Society of the United States Euthanasia Reference Manual
D
Death verification, 19, 41–44, 50, 52,
78, 80, 88
Decompression chamber, 1
Deer, 83, 85, 88
Disposal of carcasses, 45–46, 89, 90
Dogs
circulatory system, 8
injection diculties, 8–10, 13, 15, 18, 20, 23
pre-euthanasia anesthetic dosages, 33–37
restraint of, 58–59, 62–66
Donkeys, 81
Dosages
acepromazine. 35–36
calculating, 22, 26, 29–30, 34–36
for cats, pre-euthanasia anesthetics, 35
chart for Telazol & PreMix, 35
intracardiac injections, 29
intraperitoneal injections, 25–26
intravenous injections, 13, 22
for large animals, pre-euthanasia
anesthetics, 35–36, 81
ketamine, 36–37
PreMix (xylazine/ketamine)
combination, 33–35
for reptiles, pre-euthanasia anesthetics, 78
for small animals, pre-euthanasia
anesthetics, 35–36
Telazol, 34–35
xylazine, 36
“Dosing for eect”, 22
Drug cabinets, 72
E
Elk, 83
Equipment-related injuries, 67–69
Euthanasia. See also Field euthanasia;
Injections; Sodium pentobarbital
criteria for, 2–3, 34–36
death verification, 9, 41–44, 50, 52,
78, 80, 88
disposal of bodies, 45–46, 89, 90
by gunshot, 86–89
human stress, 58, 69–70
inhumane methods, 75
mass euthanasia, 90
selection criteria, 54–56
standards for, 54–56
verifying death, 41–44
volunteers and, 57
written protocol for, 54–56
Euthanasia-related stress, 69–70
Euthanasia area
cleaning considerations, 48
layout and design, 47–48
lighting for, 48
recommended equipment, 48–53
ventilation for, 48
Eye injuries, 68
Eyewash stations, 52, 68
F
Fatal-Plus, 4, 22, 26, 29
Femoral veins. See Medial saphenous veins
Feral cat box, 66
Ferrets, 77
Field euthanasia, 84–89
Firearms, 86–88
Fish, 80
G
Gas chambers, 1–2, 75, 76–77, 81
Gerbils, 76–77
Gloves, 46, 61, 63, 76–78, 80, 82
Goats, 81, 88–89
Guinea pigs, 50, 77
Gunshot
angles and points of entry, 89
for field euthanasia, 79, 83–89
H
Hamsters, 61, 76
Heart anatomy, 27
Heartbeat cessation, 13, 25, 29
Heart injections. See Intracardiac injections
Index

97
The Humane Society of the United States Euthanasia Reference Manual
Heart stick. See Cardiac stick
Holding o veins, 12, 19–20
Horses
euthanasia procedure, 10, 80–81
jugular vein location, 10
restraint of, 81, 85
shot location for field euthanasia, 88–89
use of xylazine, 36
Human safety. See also Euthanasia-related stress
accidental injections, 51, 68
animal-related injuries, 49, 67–69
equipment-related injuries, 67–69
eye injuries, 68
large animal dangers, 80–81
I
IC injections. See Intracardiac injections
Immobilization, 3, 80, 85
Inhalant anesthetics, 39–40
Injections
accidental injections to humans, 51, 68
diculties with, 10, 15–16, 23, 36–38
intracardiac, 26–29
intramuscular, 29, 31, 33–35, 37, 59
intraperitoneal, 8, 22–26, 60
intravenous, 7–22
subcutaneous, 29, 35–36
syringe preparation, 49
unacceptable injection routes, 29
Intracardiac injections
administering injection, 27–29
advantages of, 26
determining unconsciousness, 27–29
disadvantages of, 26
dosage, 29
location for, 28–29
needle size for, 28
Intrahepatic injections, 24, 29
Intraperitoneal injections
advantages of, 23
disadvantages of, 23
dosage, 25–26
locating the injection site, 24
making the injection, 24–25
post-injection care, 25–26
reaction time, 25
Intravenous injections
advantages of, 7
aspirating the syringe, 16
cephalic vein injection, 9–13
disadvantages of, 7
dosage, 13, 22
drug injection, 18–19
finding a dicult vein, 21–22
holding the syringe, 10–11
injection speed, 19, 36
inserting the needle, 14–20
jugular vein injection, 10, 20
lateral saphenous vein injection, 9–10
medial saphenous vein injection, 10
proper injection site, 12–13
removing the needle, 19
securing the syringe, 16–17, 19–20
selecting veins, 8
IP injections. See Intraperitoneal injections
IV injections. See Intravenous injections
J
Jugular vein injections, 10, 20, 81
K
Ketamine, 33–37, 71–72, 75, 80–81
L
Label dose, 6
Landfill disposal, 45, 90
Large animals
acepromazine use, 81
disposal, 45
field euthanasia, 84–89
human safety concerns, 20, 80–81
intravenous injections, 80–83
jugular vein injections, 10, 20, 81
ketamine use, 81
pre-euthanasia anesthetic dosages, 35–36, 81
sodium pentobarbital dosages, 81
syringes, 49
xylazine use, 36
Lateral saphenous vein.
See Saphenous vein injections
Lethal dose, 6
Index

98
The Humane Society of the United States Euthanasia Reference Manual
Lidocaine, 4
Livestock. See Large animals
Lizards, 78–80
M
Medial saphenous veins, 10, 13, 20
Medulla oblongata
sodium pentobarbital eects, 6
Medullary paralysis, 6, 41
Mice, 26, 76–77
Moose, 83
Mountain lions, 83
Mules, 81
Muzzles, 61–63
N
Needles
accidental wounds to humans, 51, 68
disposal of, 51
insertion into vein, 16–17, 21–22, 25
for intracardiac injections, 28
for intraperitoneal injections, 22–25
removal from vein, 18–19
sizes of, 50–51
Nye tourniquets, 13, 51
O
Occupational Safety and
Health Administration, 52
Oral administration
acepromazine, 36
sodium pentobarbital, 30, 78, 82
P
Per Os (PO), 7, 30
See also Oral administration
Pigs
euthanasia procedure, 81, 86, 88–89
restraint of, 85
Plexiglass® shields, 38, 61
Pole syringes, 38, 79
Porcupine, 82
Pre-euthanasia anesthetics
acepromazine, 35–36
administration, 37–38
advantages of, 31
disadvantages of, 31–32
dosage charts, 35
eye injuries to humans, 68
inhalant anesthetics, 39–40
injection devices, 37–38
ketamine, 36–37
licensing for, 71–74
policy, 32
PreMix, 33–37, 76–79, 83
restraint for administration of, 37–39
Telazol, 34–35
types of, 33–35
xylazine, 36
PreMix, 33–37, 76–79, 83
Primates, 83
R
Rabbits, 61, 76
Rabies, 1, 46, 67, 82–83, 87
Rabies pole. See Control pole
Raccoons, 65
Raising the vein, 12–13
Rats, 76–77
Reflexive movements, 6, 27
Reptiles, 23, 78–80
Restraint
animal handling tools, 61–66
approaches to, 58
behavior signals, 58
for cats, 60–61
concepts of, 58
control poles, 64–65
for dogs, 58–59
for field euthanasia, 85
small dog technique, 59
Rigor mortis, 41–44, 78, 80, 89
Index

99
The Humane Society of the United States Euthanasia Reference Manual
S
Safety Stick™. See Pole syringe
Saphenous vein injections, 9–10, 19–20, 52
Sedation, 3
Sharps containers, 51
Sheep,
restraint of, 85
shot location for field euthanasia, 81, 88–89
Skunks, 82
Shot locations by species, 88–89
Shotguns, 86
Small mammals
acepromazine, 36
euthanasia procedures, 76–77
intraperitoneal injections, 23–24
needle sizes, 50
PreMix, 33–35
pre-euthanasia anesthetic dosages, 34–36
xylazine use, 36
Snakes, 78–79
Snapping turtles, 79
Sodium pentobarbital.
advantages of, 4
classification of, 71
disadvantages of, 4
dosages, 13, 25, 29
for field euthanasia, 85–86
forms of, 4
inventory of, 74
intracardiac injection, 7, 26–29
intraperitoneal injection, 7, 22–26
intravenous injection, 7–21
legal requirements, 71–74
liquid form, 4, 72
method of action, 4–7
oral administration, 30
powder form, 4,72
record-keeping requirements, 73
security requirements, 72–73
state licensing regulations, 71–74
storage of, 72–73
Squeeze cage, 31, 38, 61, 65–66, 76
State laws
pre-euthanasia anesthetics licensing, 71–74,
sodium pentobarbital licensing, 71–74
Stethoscopes, 28, 41–43, 52, 80, 90
Subcutaneous (SQ), 29, 35–36
Syringes
aspirating, 17–18, 20, 25, 28–29, 43
centric, 50
locking hubs, 49–51
eccentric, 50–51
for pre-euthanasia anesthetics, 37–38
preparation of, 49
securing, 16–17, 19–20
sizes of, 49–51
T
T-61, 75
Telazol, 33–35, 60, 71, 73, 76–79, 81
Terrapins, 79
Toe pinch reflex, 41
Tortoises, 79
Tourniquets, 7, 13–14, 20, 51–52, 90
Tranquilization, 3
Turtles, 79
U
Unconsciousness, 3
determining, 27–29
Units of measure, 23
V
Ventral midline injections, 24–25, 80
Verification of death, 9, 41–44, 50, 52,
78, 80, 88
Vital functions
eect of sodium pentobarbital, 4
Voluntary excitement, 5
W
Wildlife, 81–83
X
Xylazine, 33–35, 36–37, 72–73, 81
Index

Thanks to many decades of hard work, euthanasia in animal shelters
has dramatically declined, as our field moves ever closer to eliminating
overpopulation and finding homes for all of the animals in our care.
When the dicult decision is made to euthanize an animal to alleviate
suering, it is our responsibility to ensure that euthanasia is performed as
expertly and humanely as possible. Anything less is simply unacceptable.
This manual, published by The Humane Society of the United States,
the nation’s leading animal welfare organization, is intended to serve
as the definitive reference tool for understanding the methods and
techniques of humane euthanasia. It is our hope that when there are no
lifesaving alternatives available, technicians will use this tool to provide
as humane an ending as possible for the animals entrusted to their care.
©2013 THE HSUS. ALL RIGHTS RESERVED. PRINTED ON RECYCLED PAPER.
humanesociety.orghsvma.org
